Session Information
Session Type: ACR Abstract Session
Session Time: 11:00AM-12:30PM
Background/Purpose: Lupus nephritis (LN) is a leading cause of morbidity and mortality in Systemic lupus erythematosus (SLE) patients. However, the pathogenesis of renal disease in lupus patients is not yet fully understood. CD6 is a co-stimulatory receptor, predominantly expressed on T cells, that binds to activated leukocyte cell adhesion molecule (ALCAM), a ligand expressed on antigen presenting cells (APCs) and various epithelial and endothelial tissues. The CD6/ALCAM pathway plays an integral role in modulating T cell activation, proliferation, differentiation and trafficking, and is central to immune mediated inflammation. The objectives of this research were to study the expression of CD6/ALCAM in kidneys of LN patients, and evaluate the potential of urine ALCAM as a disease biomarker in LN.
Methods: Publicly available single-cell RNA sequencing (scRNA-seq) profiles of clinically indicated renal biopsies obtained from patients with active LN were acquired. scRNA-seq analysis was performed on these datasets using the Seurat package for R. Urine samples were collected from SLE patients of multiple ethnicities and diverse disease activities. ALCAM concentrations were assayed by ELISA then normalized to urine creatinine.
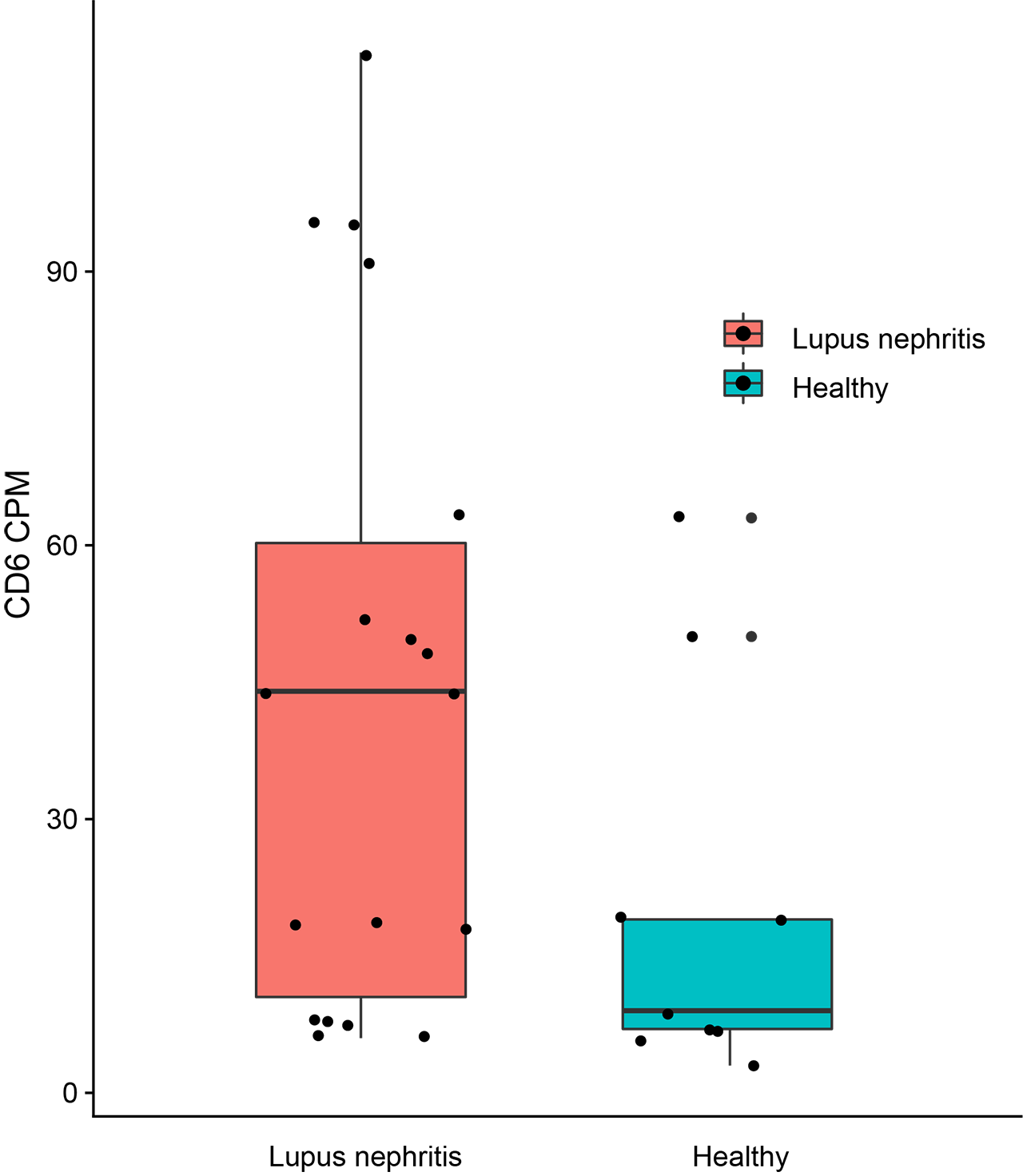
Results: Analysis of biopsies from 19 patients with LN and 11 healthy controls was performed. This analysis confirmed the expression of CD6 exclusively in T cells. ALCAM was expressed in both professional APCs such as macrophages, dendritic cells, and tubular cells. The number of CD6 expressing T cells was elevated in patients with LN compared to healthy controls (p = 0.08) (Figure 1). Patients with class III or IV LN trended towards having more CD6 expressing cells than controls. Furthermore, the number of ALCAM expressing tubular cells and macrophages were elevated in LN compared to healthy controls as well. Urine ALCAM was significantly elevated in patients with active LN when compared with controls across multiple ethnicities. In African American, Hispanic, and Asian patients, urine ALCAM further discriminated active LN from inactive SLE or active SLE patients without LN. Urine ALCAM correlated significantly with SLEDAI, rSLEDAI and PGA in Asian SLE patients (all p< 0.0001) (Figure 2).
Conclusion: Here, we demonstrate increased activity of the CD6/ALCAM pathway within the renal tissues of LN patients. More specifically, infiltrating T cells do indeed express CD6 and the number of CD6 expressing T cells are greater in renal biopsies from patients with LN vs. healthy controls, and in patients with proliferative vs. membranous LN. ALCAM expressing macrophages were also numerically elevated in patients with LN, suggesting increased activation of the CD6/ALCAM signaling pathway in LN. Patients with LN also had elevated levels of ALCAM expressing tubular cells, indicating these resident kidney cells may contribute to signaling and migration of T cells in the context of LN. Finally, urine ALCAM was significantly elevated in active LN patients in multiple ethnicities, and correlated well with clinical disease status, thus representing a promising biomarker for disease evaluation in LN. These data suggest that a targeted CD6-ALCAM therapy, such as itolizumab, may be a promising for the treatment of LN.
To cite this abstract in AMA style:
Der E, Zhang T, Mok C, Saxena R, Polu K, Mohan C, Putterman C. CD6-ALCAM Signaling Is Upregulated in Kidneys with Lupus Nephritis and Is Associated with Disease Activity [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/cd6-alcam-signaling-is-upregulated-in-kidneys-with-lupus-nephritis-and-is-associated-with-disease-activity/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/cd6-alcam-signaling-is-upregulated-in-kidneys-with-lupus-nephritis-and-is-associated-with-disease-activity/