Session Information
Date: Sunday, November 17, 2024
Title: SLE – Treatment Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Patients with Systemic Lupus Erythematosus (SLE) exhibit increased formation of Neutrophil Extracellular Traps and production of proinflammatory cytokines, which can perpetuate autoimmunity. Furthermore, NETs are associated with thromboembolic events. Recent evidence suggests that CD19 CAR T cell therapy could induce sustained, drug-free remission in autoimmune diseases including SLE. However, it is unclear how a CAR-T cell mediated immune-reset may impact the myeloid cell compartment, in particular neutrophils.
Methods: Single-cell RNA sequencing data from peripheral blood mononuclear cells (PBMCs) of five SLE patients, collected before and after CD19 CAR T cell therapy, were analyzed. Neutrophils were identified using automatic cell-type assignment via SingleR and dimensionality reduction was performed for single cells and patient-level pseudobulk expression profiles. Differentially expressed genes (DEGs) were identified using DESeq2.
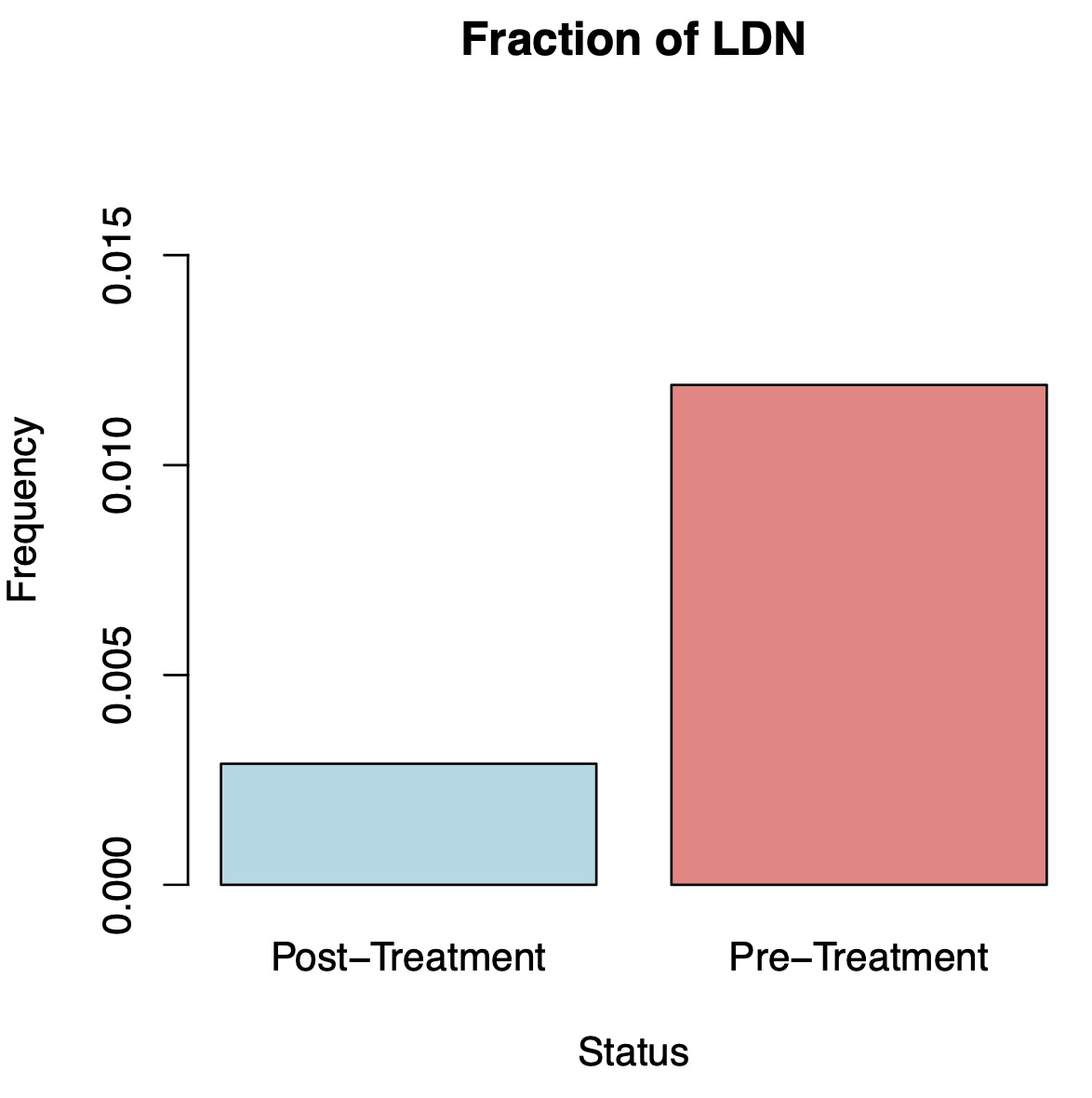
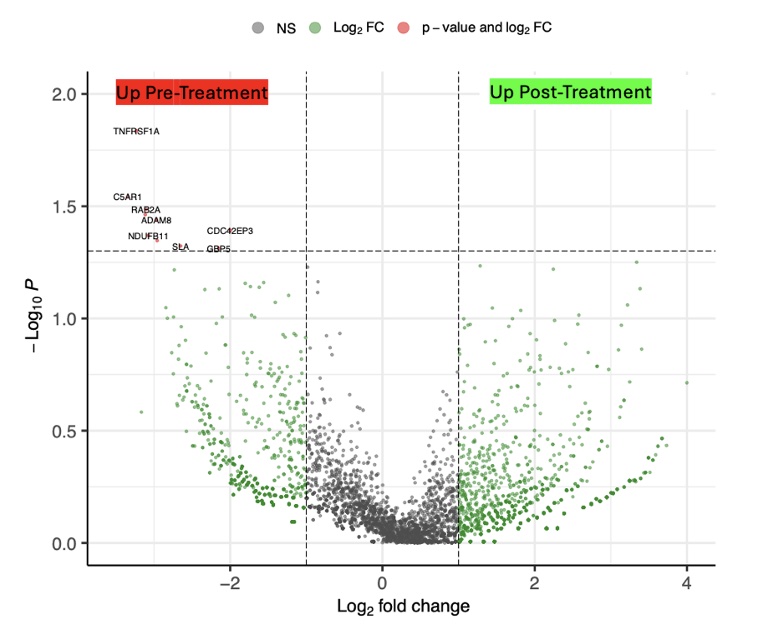
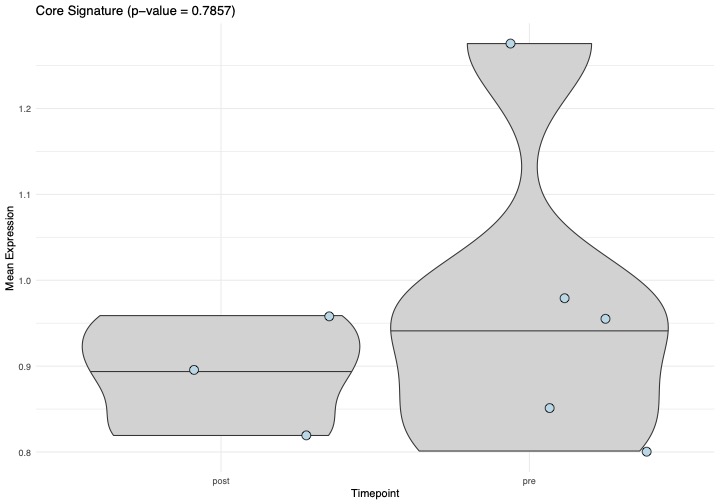
Results: At follow-up, all SLE patients were in drug free DORIS remission and had stable B cell recovery in the blood. CD19 CAR T cell treatment resulted in a five-fold reduction of the fraction of low density neutrophils in total PBMCs from 1.19 % to 0.28% (Figure 1). Within these neutrophils, we identified a total of 11 differentially expressed genes. In particular, expression of the core inflammation program, an indicator of neutrophil activation across various inflammatory conditions (2), was reduced after CAR-T cell therapy (Figure 2). The strongest reduction was observed in expression of TNFRSF1A, encoding the TNF receptor 1. Other genes with reduced expression included C5AR1 and ADAM8, which are associated with neutrophil activation and migration (Figure 3).
Conclusion: Short, deep B cell depletion via CD19 CAR-T cell therapy in SLE resulted in reduced numbers of low-density neutrophils and reduced neutrophil activation. Hence, a successful immune reset may not just reduce autoimmunity in T and B cells but also reduce inflammatory burden in the myeloid cell compartment, which could contribute to the success of the treatment and further reduce the risk for long term complications. Hence, deeper analysis of the myeloid cell compartment may inform the definition and quantification of immune reset in autoimmune disease.
To cite this abstract in AMA style:
Garantziotis P, Anoshkin K, Hagen M, Wirsching A, MAckensen A, Schett G, Grieshaber-Bouyer R. CD19 CAR T Cell Therapy Suppresses the Inflammatory Profile of Neutrophils in Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/cd19-car-t-cell-therapy-suppresses-the-inflammatory-profile-of-neutrophils-in-systemic-lupus-erythematosus/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/cd19-car-t-cell-therapy-suppresses-the-inflammatory-profile-of-neutrophils-in-systemic-lupus-erythematosus/