Session Information
Date: Wednesday, October 29, 2025
Title: Abstracts: Systemic Lupus Erythematosus – Treatment II (2693–2698)
Session Type: Abstract Session
Session Time: 11:30AM-11:45AM
Background/Purpose: CAR T-cell therapy is poised to revolutionize the treatment of systemic lupus erythematosus (SLE). Recently, several authors have reported their experience using CAR T-cell therapy for patients with SLE; nonetheless, all studies are limited by a reduced sample size. Here, we review and summarize the available clinical evidence published or presented to date on the use of CAR therapy in SLE.
Methods: We conducted a systematic review to evaluate all clinical studies assessing safety and efficacy outcomes of CAR therapy in SLE. MEDLINE (PubMed), Embase, Scopus, and CENTRAL were consulted, as well as the ACR Convergence 2024 Archives.
Results: The search strategy yielded 4,943 results, of which 14 studies were included in the analysis (including 8 from the consulted databases and 6 from the 2024 ACR Archives). The studies had a combined population of 98 participants. All studies included patients with refractory and active SLE who had failed at least 2 immunosuppressive therapies. Most studies (n=12 [85%]) used a CD19-targetted CAR, with only 2 groups (15%) assessing bispecific CD19-BCMA CAR T-cells. Two studies (15%) evaluated allogeneic CAR products. All groups reported successful B-cell depletion, typically achieved within 1–3 weeks, and B-cell reconstitution was observed after 2-3 months by most groups. Pooled analysis of the 63 individual SLEDAI scores available showed a median decrease in SLEDAI of 6 points (IQR: 3.5 – 8.5) at month 1, 8 points (IQR: 6 – 9.3) at month 3, and 8 points (IQR: 6 – 12) at month 6. The mean baseline SLEDAI of 12.8 (95% CI 11.7-13.8) decreased to 3.6 (95% CI 2.5-4.7), and 2.3 (95% CI 1.1-3.5) after 3 and 6 months; respectively. Thirty-one (48%) patients had a SLEDAI score of 0 at their last follow-up, and 59 (91%) had a score of 4 or less. There was no difference in efficacy between autologous vs allogeneic products, or between CD19-targeted vs CD19- and BCMA-targeted therapies. Cytokine release syndrome (CRS) occurred in 56 (57%) participants, of which 50 (89%) were grade 1, 5 (9%) grade 2, and 1 (2%) grade 3. Immune effector cell-associated neurotoxicity syndrome (ICANS) was reported in 3 patients (3%), 1 grade 1, 1 grade 3, and 1 grade 4. Twenty-one patients (21%) developed infections, of which six (28%) were severe. Severe (grade 3 or 4) and prolonged ( >28 days) hematologic toxicity was observed in 17 (17%) and 8 (8%) patients; respectively. Among the 5 studies analyzing B-cell phenotypes, all reported a marked increase in naïve B-cell populations (CD20+, CD27−) alongside a significant reduction in memory B-cells (CD20+, CD27+). Among the 40 patients in which serum biomarkers were measured, anti-dsDNA levels decreased in 36 (90%) patients and normalized in 29 (73%). Complement levels normalized in 37 (93%) patients.
Conclusion: This systematic review showed consistent efficacy with decrease in SLEDAI from 12.8 at baseline to 2.3 at 6 months after CAR T-cell. CRS and ICANS were milder and less frequent than expected from oncology data. The findings further support the efficacy and safety of this treatment modality while highlighting the need for additional research to better define its role in SLE management.
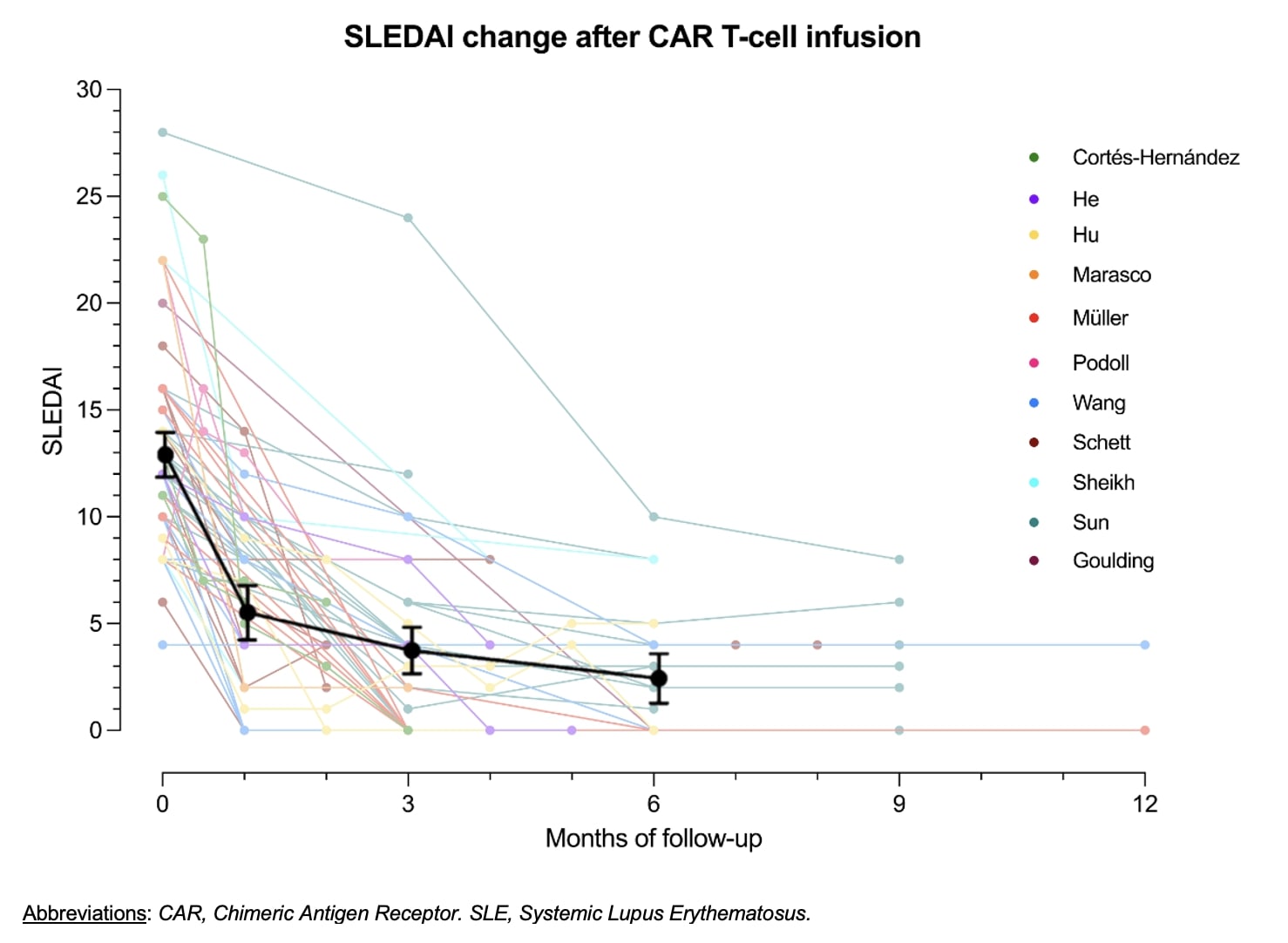 Figure 1. SLEDAI reduction in studies evaluating CAR T-cell therapy in patients with SLE
Figure 1. SLEDAI reduction in studies evaluating CAR T-cell therapy in patients with SLE
.jpg) Figure 2. Safety outcomes of CAR T-cell therapy in patients with SLE
Figure 2. Safety outcomes of CAR T-cell therapy in patients with SLE
.jpg) Figure 3. B-cell phenotypes after CAR T-cell infusion in patients with SLE
Figure 3. B-cell phenotypes after CAR T-cell infusion in patients with SLE
To cite this abstract in AMA style:
Nordmann-Gomes A, Khalili L, Tang W, Geraldino-Pardilla L, Gartshteyn Y, Clancy R, Suh S, Souvignier M, Sadelain M, Askanase A. CAR T-cell Therapy in SLE: A Systematic Review [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/car-t-cell-therapy-in-sle-a-systematic-review/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/car-t-cell-therapy-in-sle-a-systematic-review/
