Session Information
Date: Saturday, November 12, 2022
Title: Health Services Research Poster I: Lupus, RA, Spondyloarthritis and More
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: Routine management of inflammatory joint disease requires clinicians to consider an abundance of factors unique to each patient when deciding on the most appropriate course of treatment to achieve and maintain disease control, while minimising harm over an extended period. In Australia, eleven b/tsDMARDs from five drug classes are reimbursed for rheumatoid arthritis (RA) and psoriatic arthritis (PsA), and eight b/tsDMARDs from three classes for ankylosing spondylitis (AS). Treatment choice is at the discretion of the rheumatologist who also decides, in consultation with the patient, when it is no longer appropriate to continue treatment. The aim of this study was to understand the reasons why b/tsDMARDs were discontinued in routine care.
Methods: Data were sourced from the OPAL dataset, which is a collection of deidentified, aggregated clinical data derived from the electronic medical records (EMR) of 112 rheumatologists in Australia. Data is captured at the point of care in custom-built software (Audit4, S4S Pty Ltd, Sydney, Australia) which also serves as the clinician’s prescribing software. The reason for discontinuing treatment was documented by the rheumatologist in the patient’s EMR at the time of the decision from a pre-defined menu. Patients were eligible for the analysis if they were aged 18-95 years, diagnosed with RA, PsA, or AS and treated with a b/tsDMARD between Jan 2009-Mar 2022. Independent adjudication of the reason for discontinuation was not conducted.
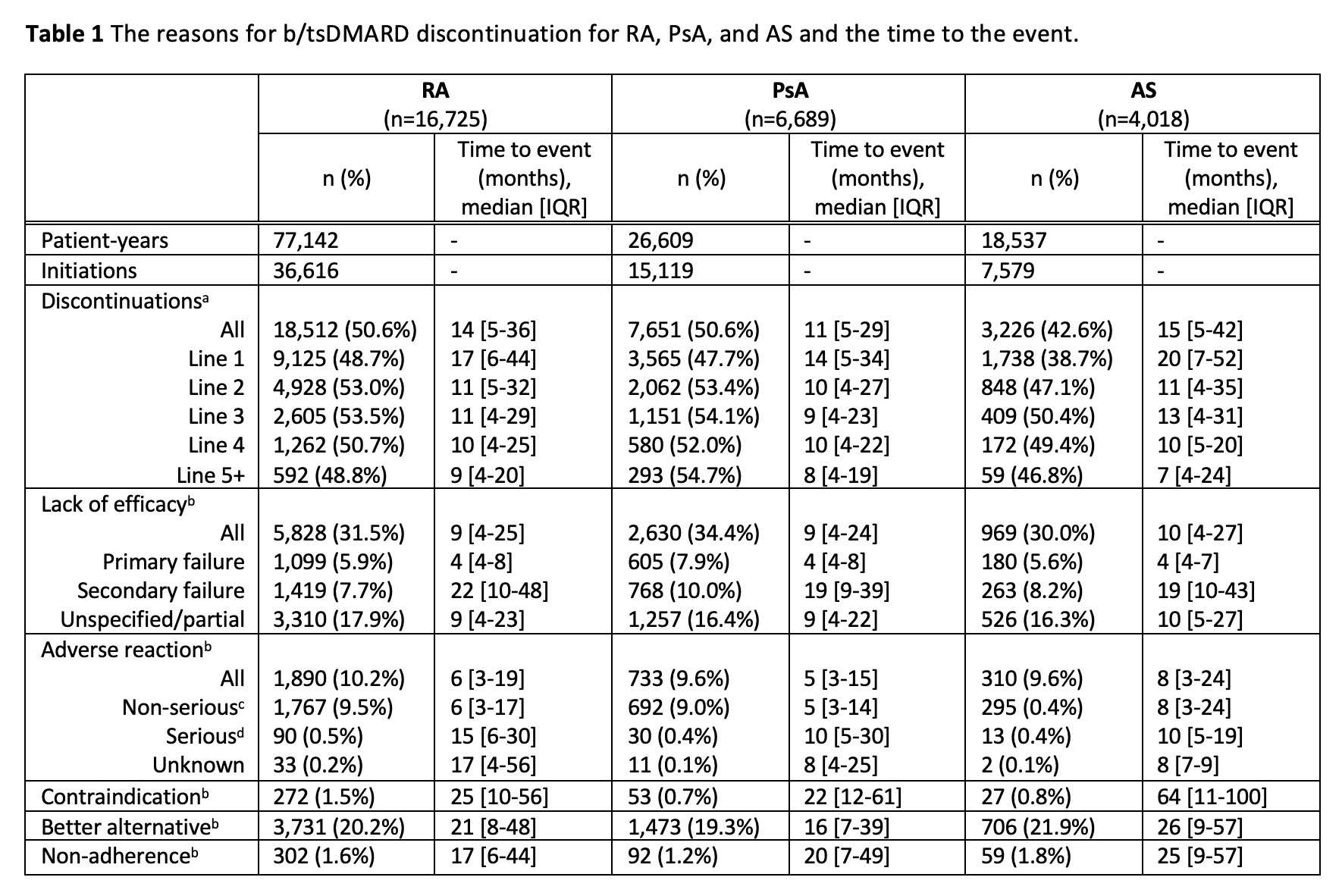
Results: 26,259 patients initiated a b/tsDMARD and were eligible for this study, and of these, 12,460 (47%) discontinued at least one b/tsDMARD. A total of 59,311 b/tsDMARD initiations were recorded, 30,287 (51%) were subsequently discontinued and a reason was captured for 24,136 (79.7%).
The median time to discontinuation was 14 months for RA, 11 months for PsA, and 15 months for AS (Table 1). The most common reason for discontinuation was lack of efficacy. Primary failure was recorded for 5.9%, 7.9% and 5.6% of discontinuations for RA, PsA, and AS respectively with a median time to discontinuation of 4 months. Unspecified lack of efficacy/partial response was recorded for 17.9% 16.4% and 16.3% of RA, PsA, and AS discontinuations, respectively at 9-10 months. Secondary failure was recorded for 7.7%, 10.0%, and 8.2% of RA, PsA, and AS discontinuations, respectively, between 19-22 months.
5.2% of all b/tsDMARD initiations for RA were subsequently discontinued due to an adverse reaction (AR), which was lower for PsA (4.8%) and AS (4.1%). Approximately 10% of all discontinuations were due to an AR, however less than 0.5% of discontinuations were due to serious ARs (classified as severe, life-threatening, hospitalization, significant or permanent disability, or death). The median time to discontinuation for non-serious ARs was 6 months, 5 months, and 8 months for RA, PsA, and AS, respectively, and 15 months for RA and 10 months for PsA and AS for serious ARs.
Conclusion: In this large dataset derived from routine care, lack of efficacy was the most common reason for discontinuing b/tsDMARDs. AR was recorded for 10% of discontinuations, however serious ARs leading to b/tsDMARD discontinuation were rarely observed.
b. Denominator is total b/tsDMARD discontinuations
c. Classified as ‘non-serious’ or ‘medically significant’
d. Classified as ‘severe’, ‘initial/prolonged hospitalization’, ‘life-threatening’, ‘persistent or severe disability’, or ‘death’
To cite this abstract in AMA style:
Littlejohn G, Smith T, Ciciriello S, Youssef P, Deakin C, Anbumurali N, OSullivan C. Capturing Clinical Reasoning in Real Time Reveals Low Rate of Serious Adverse Reactions Requiring B/tsDMARD Discontinuation in Inflammatory Joint Disease: An Analysis of the OPAL Real-World Dataset [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/capturing-clinical-reasoning-in-real-time-reveals-low-rate-of-serious-adverse-reactions-requiring-b-tsdmard-discontinuation-in-inflammatory-joint-disease-an-analysis-of-the-opal-real-world-dataset/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/capturing-clinical-reasoning-in-real-time-reveals-low-rate-of-serious-adverse-reactions-requiring-b-tsdmard-discontinuation-in-inflammatory-joint-disease-an-analysis-of-the-opal-real-world-dataset/