Session Information
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: Extensive evidence has confirmed no increased risk of cancer associated to either conventional synthetic DMARDs or anti-TNF in patients with rheumatic diseases. The risk of cancer in biologic (bDMARDs) different to anti-TNF and targeted synthetic (tsDMARDs) is considerably less investigated. As new therapies are emerging, more data in real-world registries are needed to confirm safety in other treatment groups. Our objective was to compare the risk of cancer of tsDMARDs and other bDMARDs versus anti-TNF in patients with rheumatic diseases.
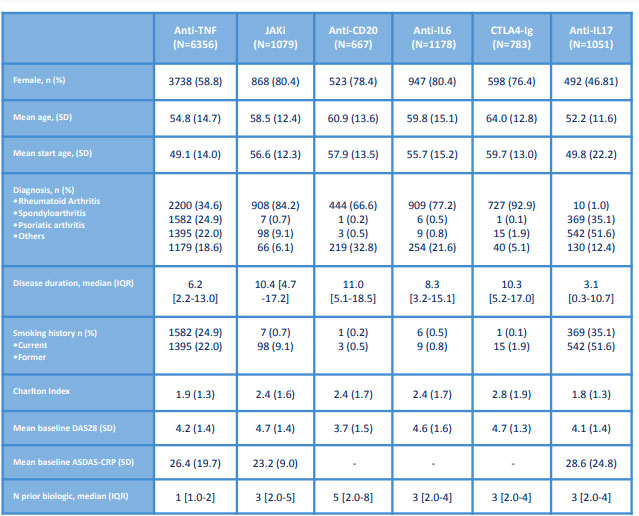
Methods: Data of patients enrolled in the biologics register BIOBADASER 3.0 up to October 2021 with the start of any bDMARD or tsDMARD were analyzed. For each group, demographic and clinical variables were estimated. Changes to therapy and occurrence of serious adverse events collected annually. Incident cancer was defined as any cancer during the exposure classified according to Meddra dictionary (version 19.0) leading to therapy discontinuation. Incidence rate ratios of cancer per 1000 patients-year (PYs) and 95% confidence interval were estimated. Incidence rate ratio was calculated to compare risk for each group versus anti-TNF.
Results: We identified 271 cancers in BIOBADASER 3.0, corresponding to a cancer incident rate of 7.4 (6.5-8.3) per 1000 PY of exposure. Patients exposed to anti-TNF and anti-IL17 were younger, with lower disease duration and comorbidity vs other groups. Proportionally more malignancies were identified in the CTLA-4 Ig group (3.4%) versus the anti-TNF group (2.9%). The interval between initiation of therapy and cancer was similar between groups. The rates of incident cancer ranged between 2.6 events/1000 PY in the anti-IL17 group and 15.3 events/1000 PY in the CTLA-4 Ig group. The rate of cancer did not differ significantly in patients exposed to JAKi [0.8 (95% CI 0.4-1.5)], anti CD20 [1.1 (95% CI 0.6-1.8)], or anti-IL6 [1.3 (95% CI 0.9-1.9)] versus anti-TNF; it was significantly lower in patients exposed to anti-Il17 [0.4 (95% CI 0.2-0.9)], and significantly higher in CTLA-4 Ig [2.2 (95% CI 1.4-3.2)]. The most frequent malignancy was non-melanoma skin cancer, followed by solid cancer (breast cancer and lung cancer).
Conclusion: Rates of incident cancer did not differ between patients treated with anti-TNF and other bDMARDs or tsDMARDs, with the possible exception of a potential increased risk in patients treated with anti-CTLA-4, but they were older and presented higher comorbidity.
To cite this abstract in AMA style:
Castrejon I, Molina J, Perez-Garcia C, Vela-Casampere P, Diaz-Torne C, Bohorquez C, Blanco-Madrigal J, Sanchez-Alonso F. Cancer Risk in Patients with Rheumatic Diseases Exposed to Different Biologic and Targeted Synthetic DMARDs in Real World Clinical Practice: Data from BIOBADASER [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/cancer-risk-in-patients-with-rheumatic-diseases-exposed-to-different-biologic-and-targeted-synthetic-dmards-in-real-world-clinical-practice-data-from-biobadaser/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/cancer-risk-in-patients-with-rheumatic-diseases-exposed-to-different-biologic-and-targeted-synthetic-dmards-in-real-world-clinical-practice-data-from-biobadaser/