Session Information
Date: Monday, November 13, 2023
Title: (1052–1081) Immunological Complications of Medical Therapy Poster
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Immune checkpoint inhibitors (ICI) have revolutionized the treatment of cancer. A drawback of ICI therapy is their off-target effects known as immune-related adverse events (irAEs), including inflammatory arthritis (ICI-IA). Glucocorticoids (GC) are among the first-line treatment for most irAEs, including ICI-IA. However, given the immunosuppressive properties of GC, there is concern that they may blunt the anti-tumor effects of ICI. There is a paucity of evidence on the safety of GC to treat ICI-IA. The aim of this study was to examine the impact of dose, timing of initiation and duration of GC on cancer outcomes in patients with ICI-IA. We hypothesized that highmaximal dose, early initiation and prolonged duration of GC exposure after onset of ICI-IA would be associated with worse outcomes.
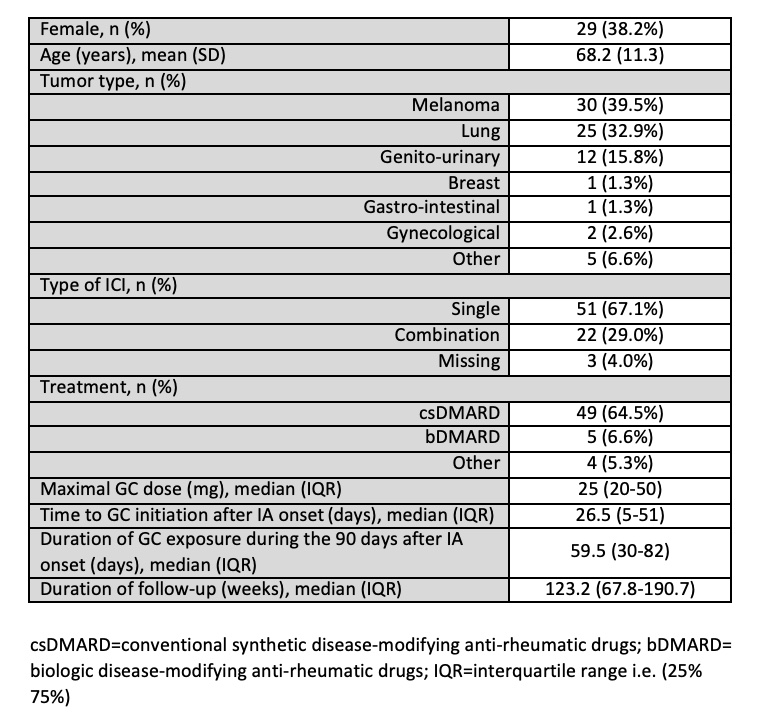
Methods: The CanRIO retrospective cohort includes adult subjects with rheumatic irAEs after exposure to ICI, seen at one of 10 rheumatology clinics across Canada between Jan 2013 and Aug 2022, who have had standardized chart data extraction. In this sub-study, participants with de novo ICI-IA (without pre-existing IA), who were exposed to GC within 90 days after onset of IA, and in whom there was at least 3 months of follow up after onset of ICI-IA were analyzed. Data on maximal GC dose (max-GC dose), timing of GC initiation (time-GC) and duration of GC exposure (dur-GC) during the 90 days after the onset of ICI-IA were extracted. Median values for each were determined and used to dichotomize the exposure variables into above and below the median. Kaplan Meier analysis were used to evaluate the association between max-GC, time-GC and dur-GC with progression-free survival and overall survival.
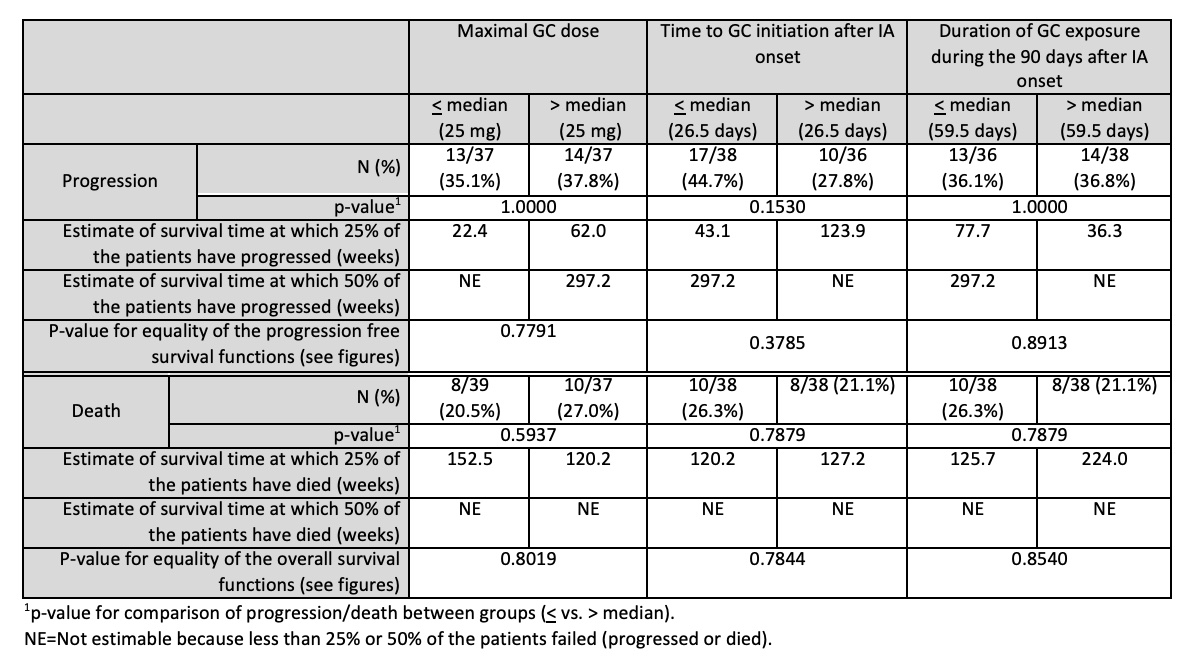
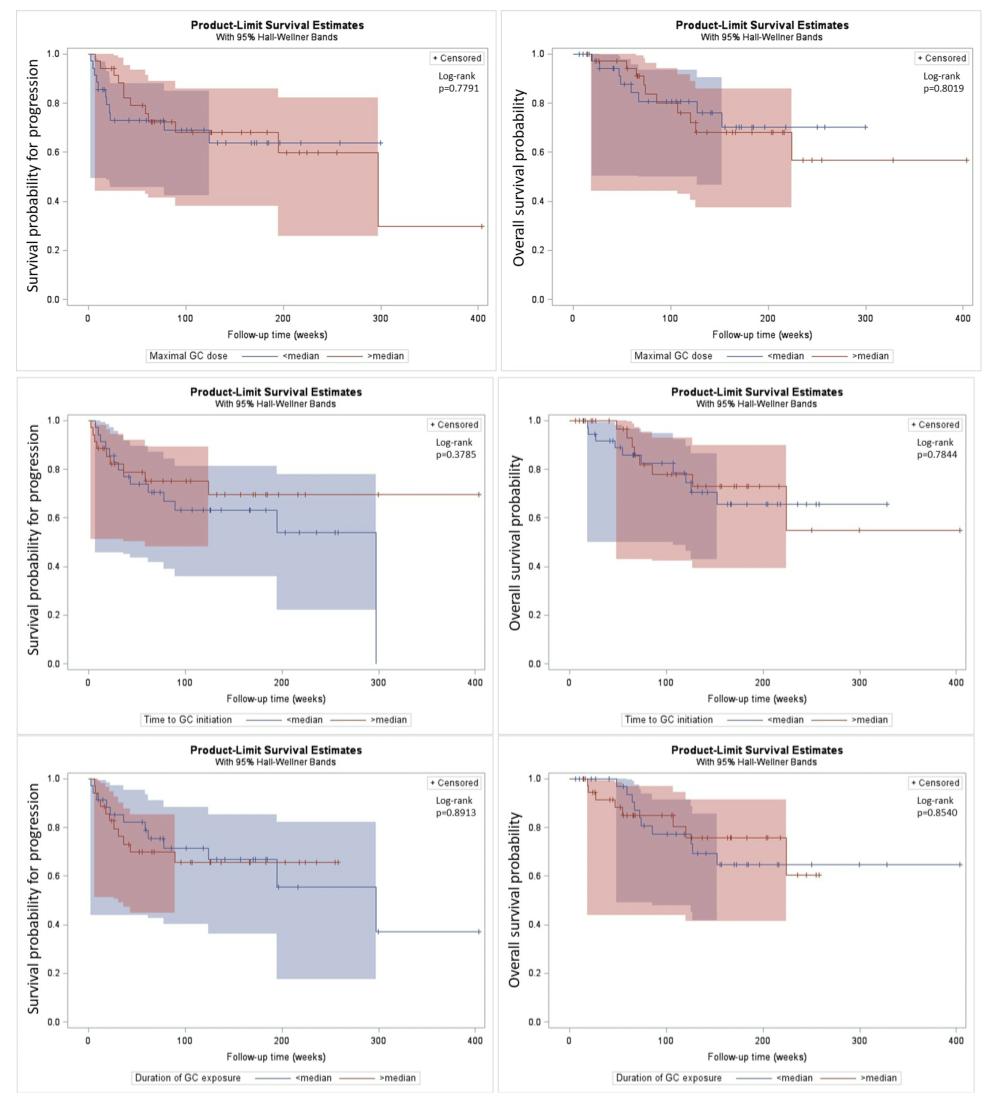
Results: Data on 76 participants with de novo ICI-IA were available for analysis (Table 1). The median max-GC dose was 25 mg daily (interquartile range (IQR) 20-50 mg), median time-GC was 26.5 days (IQR 5-51 days) and median dur-GC was 59.5 days (IQR 30-82 days). Median follow up of the participants was 123.2 weeks. Max-GC dose, time-GC and dur-GC exposure had no association with progression-free and overall survival (Table 2 and Figure 1).
Conclusion: In this study of participants with de novo ICI-IA who were treated with GC, dose of GC, timing of initiation of GC and duration of GC exposure after onset of ICI-IA did not impact tumour outcomes. Although the confidence intervals were wide, our findings provide some reassurance about the safety of short-term GC for the management of ICI-IA. Further studies with larger sample size and longer term follow up are needed to corroborate our results, and additional studies comparing to ICI-IA patients unexposed to GC are needed to optimize management.
To cite this abstract in AMA style:
Savarimuthu V, Ladouceur A, Terry O, Roberts J, Pope J, Appleton T, Hoa S, Fifi-Mah A, Maltez N, Saltman A, Himmel M, Colmegna I, Cho L, Schmidt E, Berger C, Barnetche T, Gonzalez Arreola L, Ye C, Jamal S, Hudson M. Cancer Outcomes in Cancer Patients with Immune Checkpoint Inhibitor-induced Inflammatory Arthritis Treated with Glucocorticoids: Data from the CanRIO Retrospective Cohort [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/cancer-outcomes-in-cancer-patients-with-immune-checkpoint-inhibitor-induced-inflammatory-arthritis-treated-with-glucocorticoids-data-from-the-canrio-retrospective-cohort/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/cancer-outcomes-in-cancer-patients-with-immune-checkpoint-inhibitor-induced-inflammatory-arthritis-treated-with-glucocorticoids-data-from-the-canrio-retrospective-cohort/