Session Information
Date: Sunday, November 10, 2019
Title: Pediatric Rheumatology – ePoster I: Basic Science, Biomarkers, & Sclerodermic Fever
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
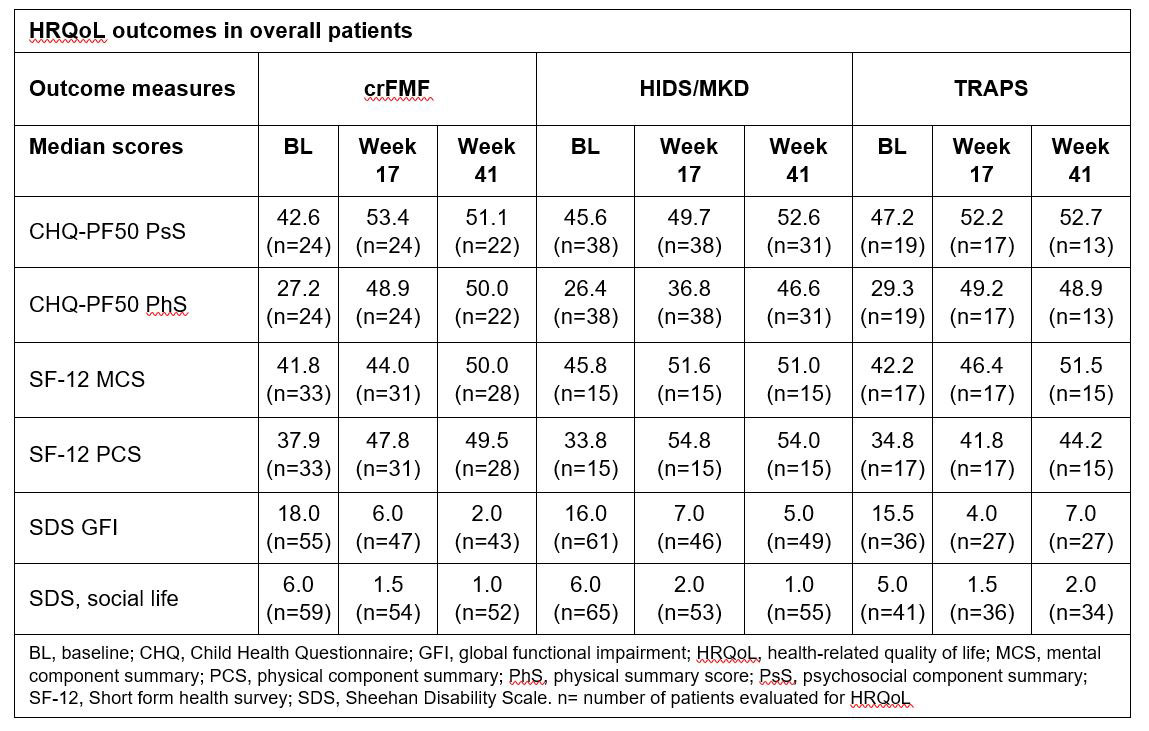
Background/Purpose: Recurrent fever syndromes have a significant impact on health-related quality of life (HRQoL).1 Canakinumab (CAN) has demonstrated efficacy and safety in patients with colchicine-resistant familial Mediterranean fever (crFMF), hyper-immunoglobulin D syndrome/mevalonate kinase deficiency (HIDS/MKD) and tumor necrosis factor receptor-associated periodic syndrome (TRAPS) in the pivotal phase 3, CLUSTER trial (NCT02059291).2 However, the published data on impact of CAN on the HRQoL, work/school and social life of these patients are limited. To evaluate the effect of CAN on HRQoL, work/school and social life of patients in the 3 disease cohorts (crFMF, HIDS/MKD, and TRAPS) in a double blinded randomized Phase 3 trial (CLUSTER).
Methods: The detailed study design was reported previously.2 The HRQoL of patients treated with CAN who met the primary endpoint of the CLUSTER trial2 was assessed at Baseline (BL), Week 17 (Wk17) and Week 41 (Wk41). HRQoL was also assessed in all patients (those initially randomized to CAN, those randomized to placebo who subsequently switched to CAN and those who did not switch to CAN). Outcomes measures were the Child Health Questionnaire (CHQ)-PF50 psychosocial (PsS) and physical (PhS) component summary scores (children >5–< 18 years), Short-Form health survey (SF-12) physical component summary (PCS) and mental component summary (MCS; adults ≥18 years) scores. An increase from baseline of 2, 5, and 8 points in the CHQ-PF50 PsS and PhS corresponds to a small, moderate and large treatment effect, respectively. Functional impairment related to work/school, social life and family life/home responsibilities was assessed by Sheehan Disability Scale (SDS) with a score of 5 or higher being associated with significant impairment. Results: Out of 181 patients, 90 (31 crFMF, 37 HIDS/MKD, and 22 TRAPS) were randomized to CAN treatment and 91 to placebo. Patients in all 3 cohorts showed a high impairment of HRQoL at baseline. In patients who met the primary endpoint, CAN was shown to improve CHQ-PF50 PsS and PhS; SF-12 MCS and PCS; and SDS scores, from baseline to Wk 17, which was maintained at Wk 41 (Table 1). The improvement in HRQoL was also observed in all patients treated with CAN, and was sustained through Wk 41 (Table 2).
Conclusion: Treatment with canakinumab led to sustained improvement of HRQoL, work/school and social life in patients with crFMF, HIDS/MKD and TRAPS.
References:
- Sahin et al. Eur Rev Med Pharmacol Sci. 2013;17:958–963.
- De Benedetti et al. NEJM 2018;378:1908–1990.
To cite this abstract in AMA style:
Lachmann H, Lauwerys B, Miettunen P, Kallinich T, Jansson A, Rosner I, Manna R, Murias S, Savic S, Smeets S, De Benedetti F, Simon A. Canakinumab Improves Patient-Reported Outcomes in Patients with Recurrent Fever Syndromes: Results from a Phase 3 Trial [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/canakinumab-improves-patient-reported-outcomes-in-patients-with-recurrent-fever-syndromes-results-from-a-phase-3-trial/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/canakinumab-improves-patient-reported-outcomes-in-patients-with-recurrent-fever-syndromes-results-from-a-phase-3-trial/