Session Information
Date: Monday, November 18, 2024
Title: Abstracts: SLE – Treatment II: Non-Cellular Lupus Therapeutics
Session Type: Abstract Session
Session Time: 1:00PM-2:30PM
Background/Purpose: The latest guidelines for managing lupus nephritis (LN), including the 2024 KDIGO guidelines and the 2019 EULAR/ERA-EDTA update, recommend a minimum immunosuppression (IS) duration of 36 months. These guidelines suggest that patients with a sustained complete renal response (CRR) may consider gradually tapering off the therapy after 3 to 5 years, although the optimal duration of treatment remains undetermined and should be tailored to individual needs. Despite current clinical practices, a significant rate of renal relapse (RR) persists, ranging from 10% to 50% following the discontinuation of IS therapy. This study aims to investigate the rate and factors associated with RR in patients with LN who have discontinued IS therapy due to a sustained CRR after a minimum of three years of treatment.
Methods: This monocentric, retrospective, observational study identified patients with LN confirmed by renal biopsy, from July 1972 to September 2023. Out of the 272 diagnosed patients, those who had discontinued IS therapy due to a sustained CRR after at least 36 months of treatment were selected for the analysis.
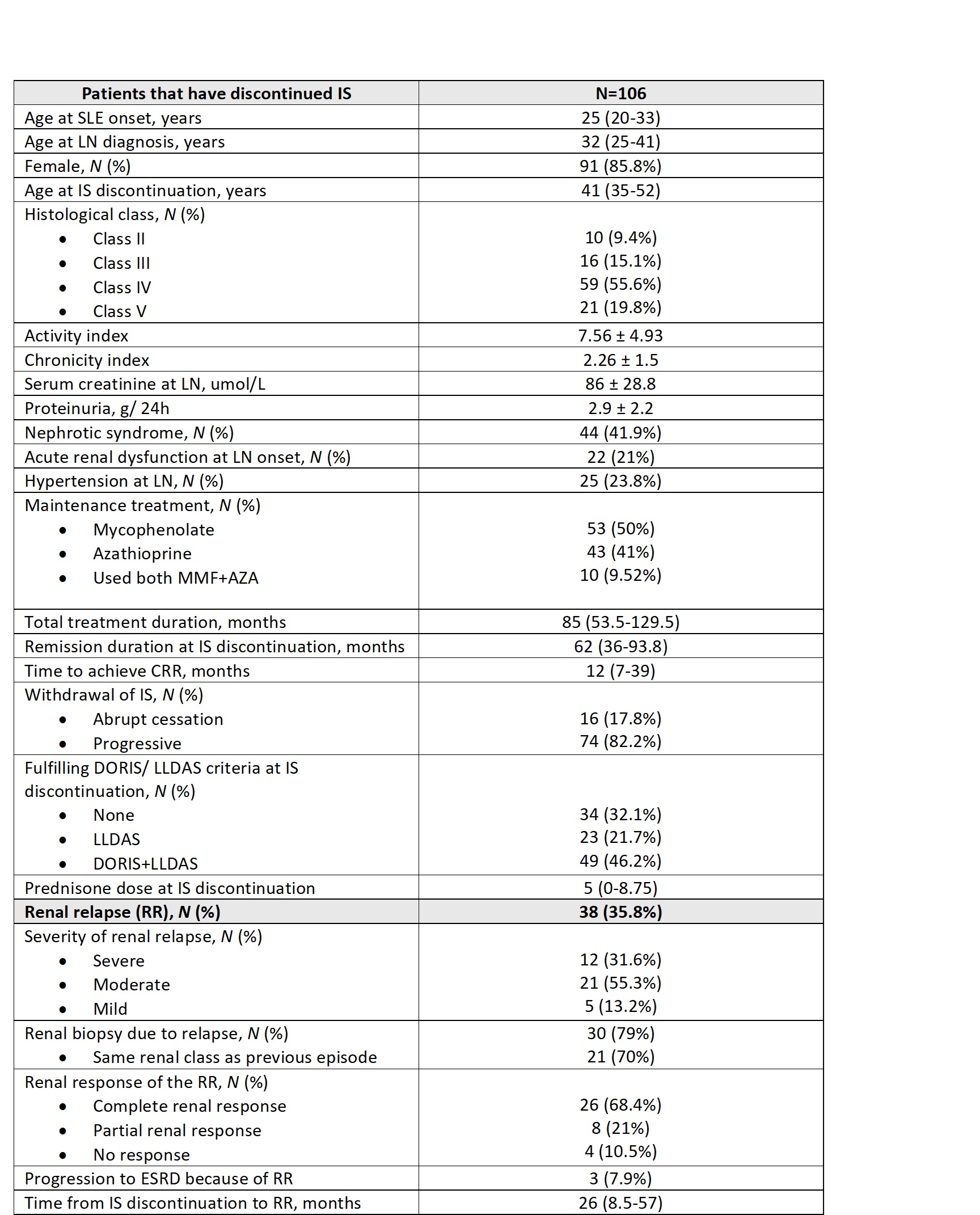
Results: A total of 106 patients (91 women) were included in the study, with a mean age of 33.6 ± 12 years at the time of LN diagnosis. Their main characteristics are summarized in Table 1. The median follow-up duration was 18.25 years from LN diagnosis and 9.6 years from IS discontinuation. Regarding LN classification at diagnosis, 9.4% were class II, 15.1% class III, 55.6% class IV, and 19.8% class V. The median duration of IS therapy before discontinuation was 85 months.
Among these patients, 38 (35.8%) experienced a RR after a median of 26 months following IS discontinuation. Of these, 30 patients (79%) underwent a renal biopsy during the relapse, with 70% retaining the same renal class as their initial diagnosis. Following the relapse, 26 patients (68.4%) achieved a CRR, 8 patients (21%) attained a partial response, and 3 patients (7.9%) progressed to end-stage renal disease (ESRD).
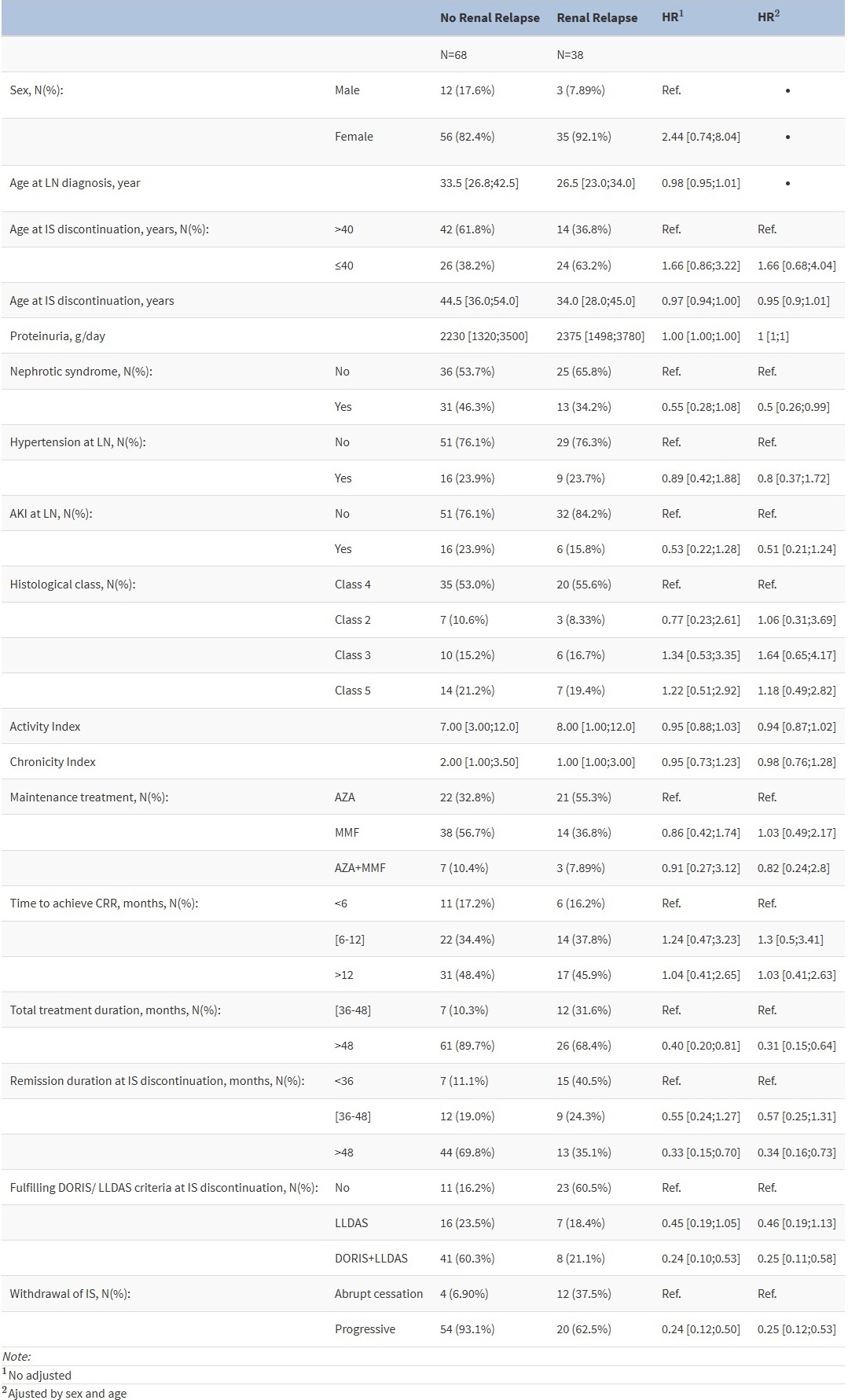
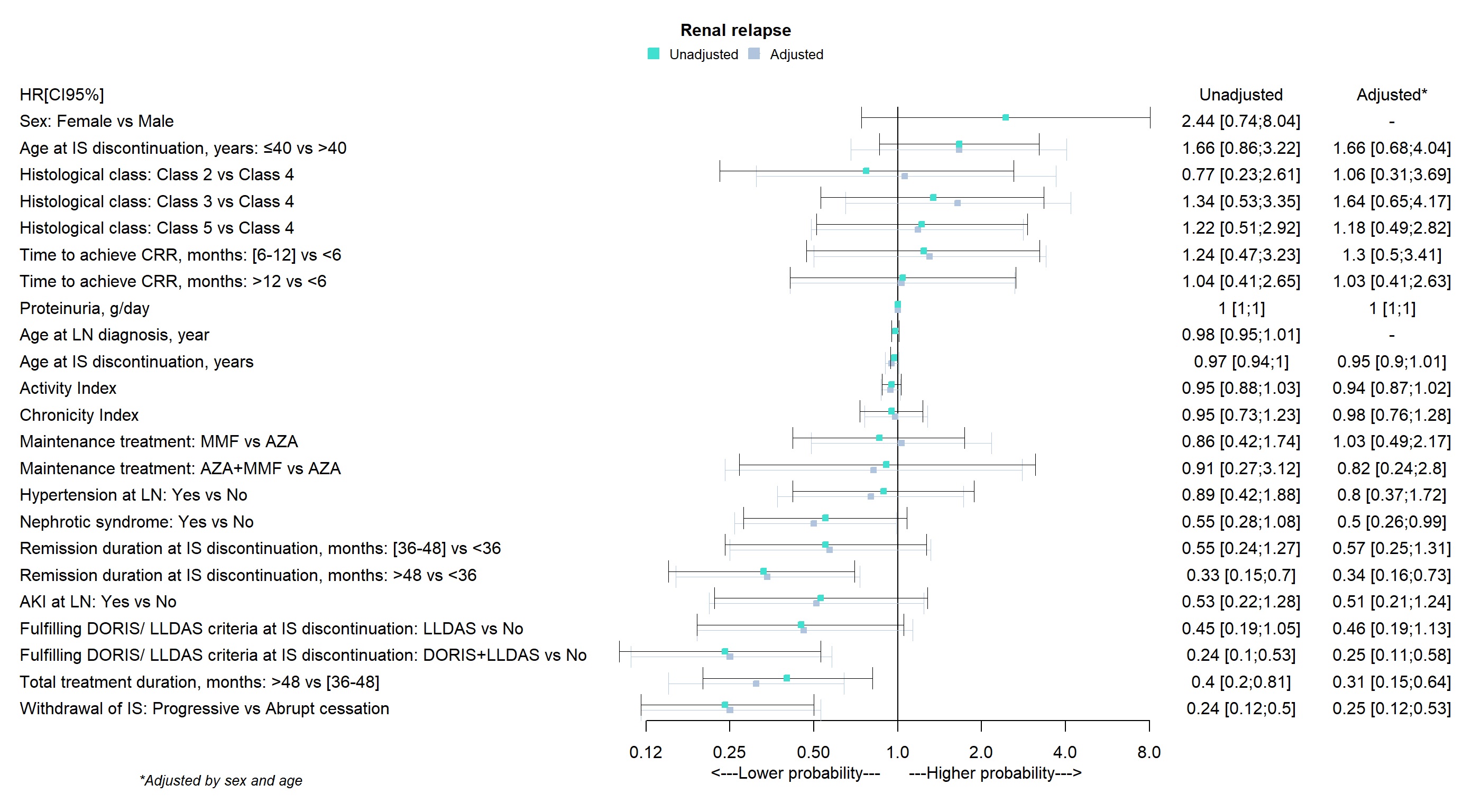
Factors Influencing Renal Relapse (RR): As shown in Table 2 and Figure 1, the following factors are associated with a reduced risk of relapse: 1) extending total IS therapy beyond 48 months, with a hazard ratio (HR) of 0.31 (95% CI: 0.15 – 0.64); 2) maintaining a complete renal response (CRR) for more than 48 months before discontinuing treatment (HR: 0.34; 95% CI: 0.16-0.73); 3) meeting clinical remission criteria as defined by the DORIS 2021 guidelines at the time of IS discontinuation (HR: 0.25; 95% CI: 0.11 – 0.58); and 4) gradually discontinuing IS therapy (HR: 0.25 compared to abrupt discontinuation; 95% CI: 0.12 – 0.53).
Conclusion: Our findings suggest that discontinuation of therapy should be carefully considered and limited to specific cases. Patients eligible for discontinuation should have received IS therapy for at least four years and maintained a CRR for over four years. Furthermore, these patients must show quiescence of extrarenal SLE manifestations in accordance with DORIS criteria at the time of IS discontinuation. Additionally, the tapering off of IS should be gradual to optimize outcomes.
To cite this abstract in AMA style:
Vidal P, Narvaez-García J, Fulladosa F, Mitjavila F, Capdevila o, Maymó P, Palacios J, Nolla J. Can Immunosuppressive Therapy Be Safely Discontinued in Patients with Lupus Nephritis? [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/can-immunosuppressive-therapy-be-safely-discontinued-in-patients-with-lupus-nephritis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/can-immunosuppressive-therapy-be-safely-discontinued-in-patients-with-lupus-nephritis/