Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: The Systemic Lupus International Collaborating Clinics (SLICC), Lupus Foundation of America (LFA), and American College of Rheumatology (ACR) are leading a global initiative to create a revised Systemic Lupus Erythematosus Damage Index (SDI). One limitation of the current SDI is a notable floor effect where most patients score between zero and three, with up to 50% in most cohorts scoring zero.1 Potential ways to address this floor effect include adding items and/or providing more sensitive definitions through describing different grades within the same item. This study aims to report the items suggested for gradation in a revised damage index.
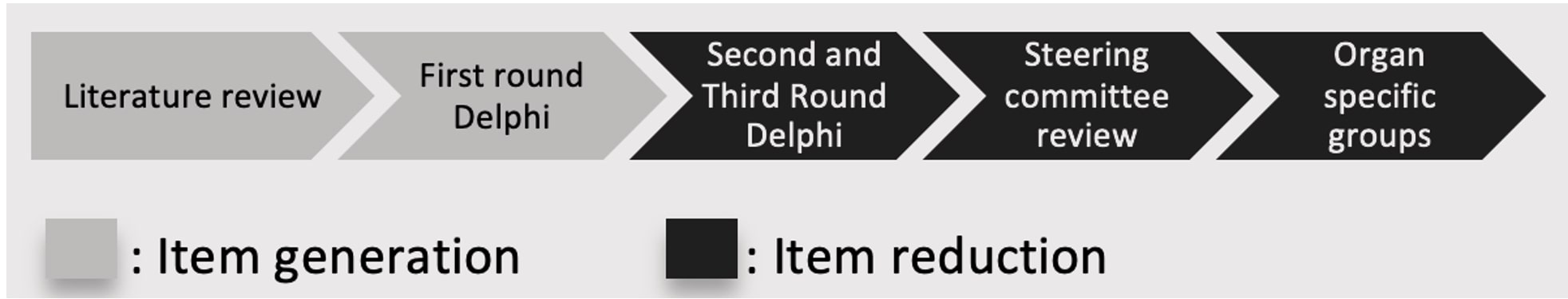
Methods: Item generation began with a comprehensive literature review and an initial Delphi round. Item reduction involved conducting two additional Delphi rounds, where items with a median score of ≤4 out of 9 were excluded. Following this, a 14-member steering committee assessed the remaining items and removed those that did not reflect the damage construct, were excessively rare, or were not feasible to assess. The expert organ domain groups then refined the remaining items, suggesting severity gradations for relevant items. Figure 1 illustrates the stages of item generation and reduction.
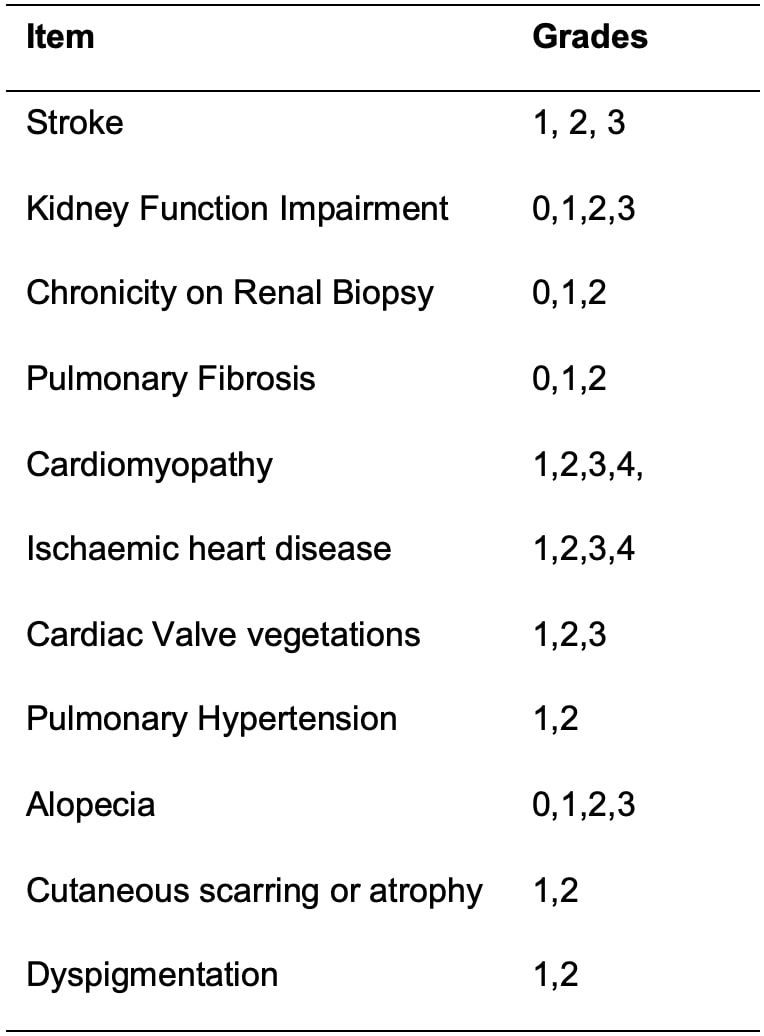
Results: The item generation and reduction phases resulted in 45 proposed candidate items for the revised SDI.2 Of these items, organ-specific groups suggested grading for 11 (24.4%) items (Table 1). For example, ischaemic heart disease is proposed to be graded as follows: Grade 1: asymptomatic ischaemia on testing, Grade 2: symptomatic angina with confirmatory tests Grade 3: myocardial infarction (clinical presentation and confirmatory tests) and Grade 4: multiple myocardial infarction and/or coronary artery bypass grafting. Similarly, kidney function impairment is proposed to be graded according to Kidney Disease Improving Global Outcomes (KDIGO) categories of glomerular filtration rate (GFR). Grade 0 -GFR ≥60 mL/min/1.73 m², Grade 1 -GFR 30-59 mL/min/1.73 m², Grade 2 – GFR 15-29 mL/min/1.73 m², and Grade 3 GFR < 15 mL/min/1.73 m².
Conclusion: Grading damage items is possible for 24.4% of proposed items in a new revised damage index. This offers a more detailed and clinically relevant assessment of organ damage in SLE patients. It reflects current evidence based medical practice and is likely to improve sensitivity of the index in SLE populations. Further validation in cohorts and consideration of weighting of grades across clinical organ systems is now underway.
< ! Sutton EJ et al. SLICC damage index: a systematic literature review. Seminars in arthritis and rheumatism. 2013; 43(3):352-361
< ! Kundakci B et al. POS1139 Lupus Damage Index Revision – Item Generation and Reduction Phase. Annals of the Rheumatic Diseases. 2024; 83:1009-1010.
To cite this abstract in AMA style:
Kundakci B, Barber M, Clarke A, Johnson S, Bruce I, organ damage index (SDI) collaborators O. Can Damage Items Be Graded According to Severity in a Revised Organ Damage Index for Lupus? [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/can-damage-items-be-graded-according-to-severity-in-a-revised-organ-damage-index-for-lupus/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/can-damage-items-be-graded-according-to-severity-in-a-revised-organ-damage-index-for-lupus/