Session Information
Date: Wednesday, October 24, 2018
Title: 6W022 ACR Abstract: Muscle Biology, Myositis & Myopathies II: Clinical & Misc Topics (2976–2981)
Session Type: ACR Concurrent Abstract Session
Session Time: 11:00AM-12:30PM
Background/Purpose: Treatment options for myositis-associated interstitial lung disease (ILD) include corticosteroids (CS) in combination with or without cyclophosphamide (CY), calcineurin inhibitors (CNIs) such as cyclosporine (CyA) and tacrolimus (TAC), mycophenolate mofetil, and/or rituximab. However, efficacy of individual treatment regimens has never been compared in randomized clinical trials, and treatment decision is made based on expertise of physicians. We have recently launched the multicenter retrospective cohort of Japanese Patients with Myositis-associated ILD (JAMI), which involved 497 incident cases of adult myositis-associated ILD from 44 institutions across Japan. By taking advantage of our large-scale cohort database, we investigated the efficacy of CNIs in myositis-associated ILD.
Methods: A total of 491 patients with adult-onset PM/DM were selected from the JAMI database. Clinical characteristics and survival rates were compared between the patient groups, followed by additional comparisons using propensity score matching method. Survival rates were calculated by the Kaplan-Meier method.
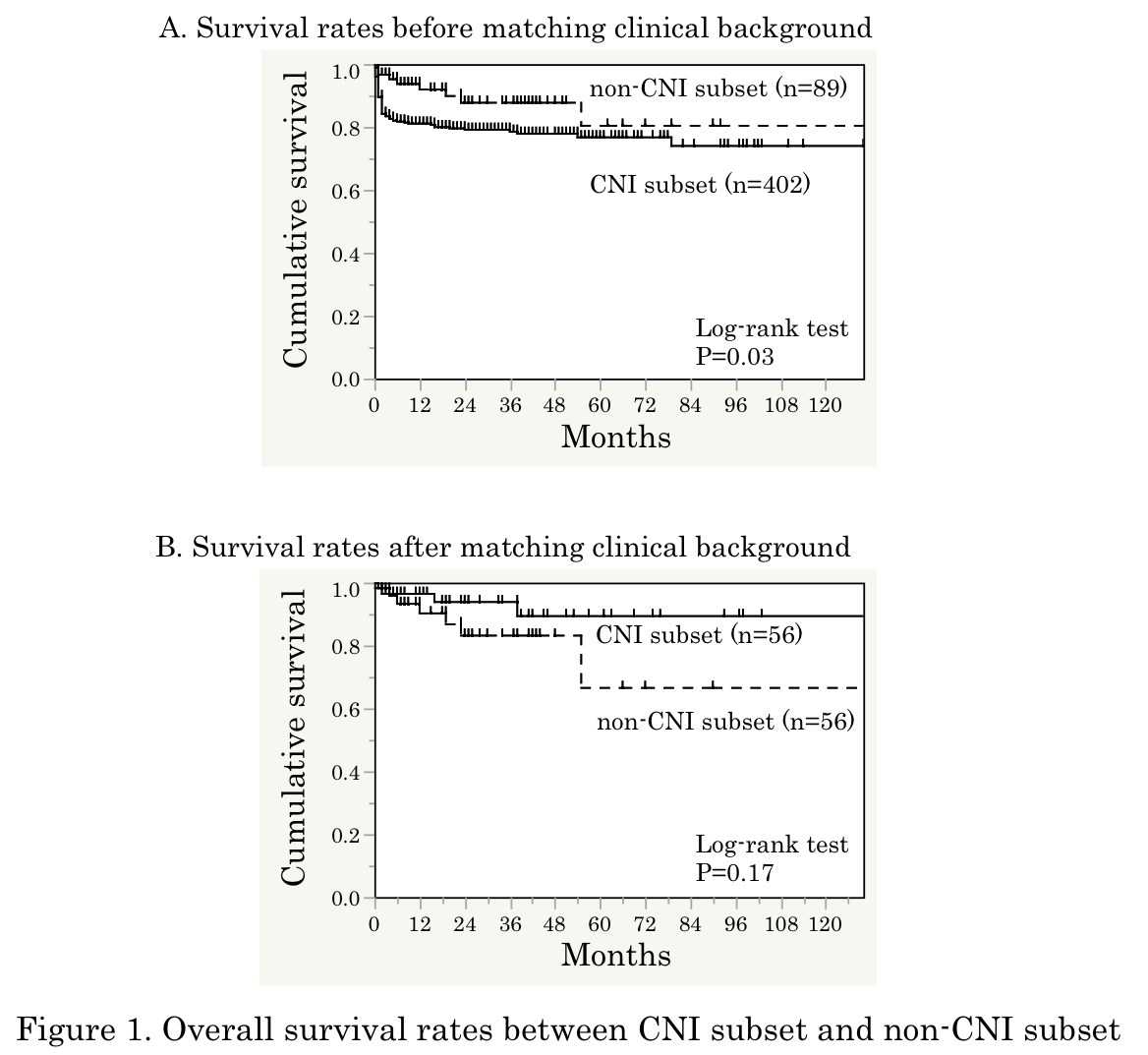
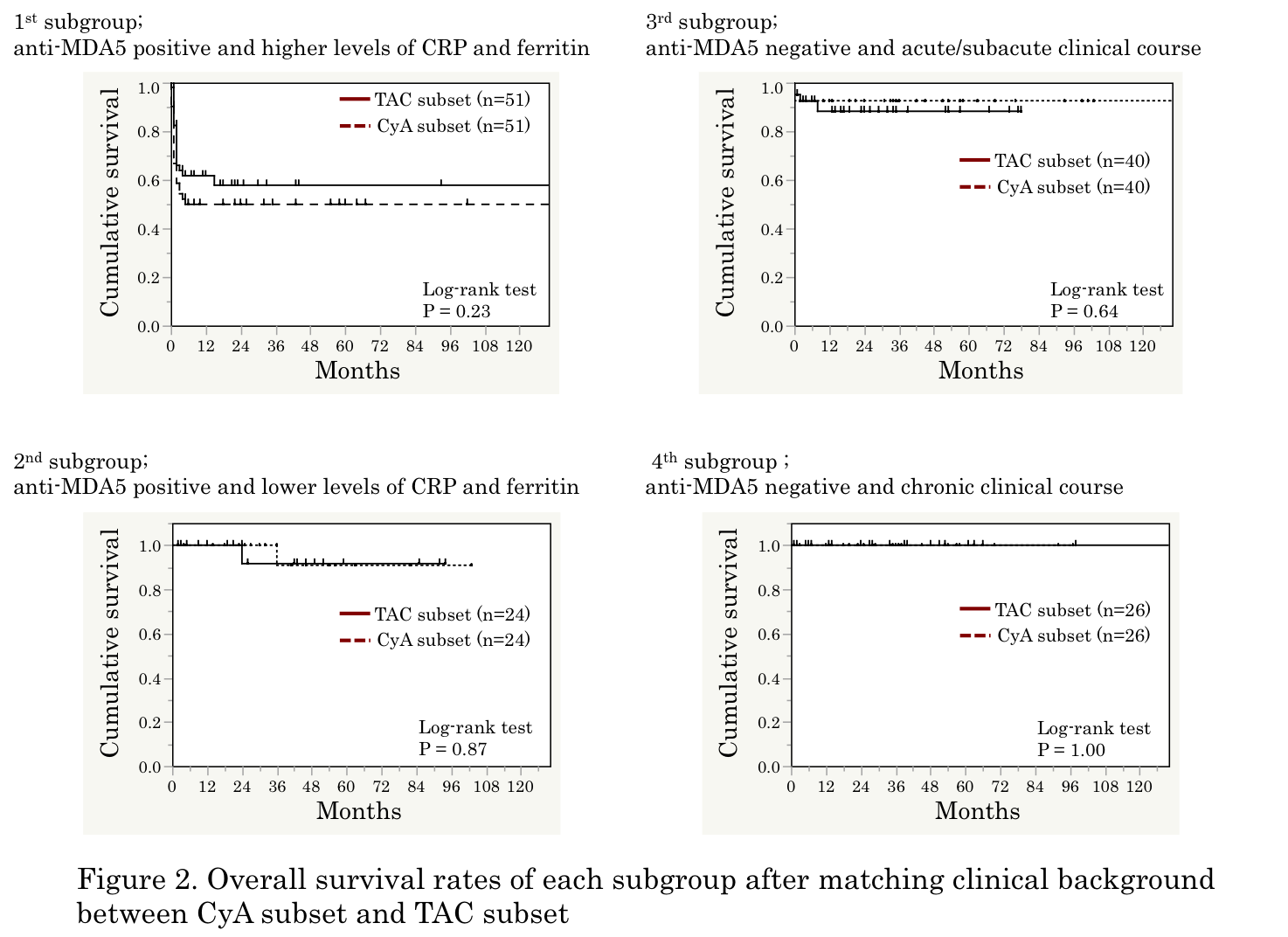
Results: The mean age at disease onset was 56, 67% were female, and median disease duration was 2 months. In a crude analysis, cumulative survival rate was significantly lower in 402 patients treated with regimens including CNI than in 89 patients treated with regimens without CNI (P = 0.03; Figure 1A). Clinical characteristics associated with CNI use included positive anti-MDA5, shorter disease duration, higher levels of CRP and ferritin, lower oxygen saturation, and more intensive immunosuppressive regimens. After matching patientsf background using propensity score, there was a trend toward a better survival rate in patients treated with CNI than in those without (Figure 1B). When we further examined efficacy between 201 patients treated with CyA and 187 patients with TAC, treatment with TAC was associated with a better survival rate, compared with CyA treatment, in a crude analysis (P = 0.02). After matching patientsf background using propensity score, the survival rates were similar between patients treated with CyA and TAC. The patients treated with CNIs were further divided into 4 subgroups according to main components contributing to the propensity score, including anti-MDA5, CRP, ferritin, and disease duration at diagnosis, there was no difference of survival rates between CyA-treated and TAC-treated patients in each subgroup (Figure 2).
Conclusion: Our results suggest potential efficacy of CNI, either CyA or TAC, in combination with CS-based regimens for broad spectrum of myositis-associated ILD.
To cite this abstract in AMA style:
Gono T, Masui K, Nishina N, Sato S, Kuwana M. Calcineurin Inhibitor for the Treatment of Myositis-Associated Interstitial Lung Disease: Comparison between Cyclosporine and Tacrolimus [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/calcineurin-inhibitor-for-the-treatment-of-myositis-associated-interstitial-lung-disease-comparison-between-cyclosporine-and-tacrolimus/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/calcineurin-inhibitor-for-the-treatment-of-myositis-associated-interstitial-lung-disease-comparison-between-cyclosporine-and-tacrolimus/