Session Information
Session Type: Late-Breaking Abstract Session
Session Time: 10:30AM-10:45AM
Background/Purpose: National U.S. data on breakthrough COVID-19 infection in people with autoimmune or inflammatory rheumatic diseases (AIRD) are limited. Our objective was to assess whether breakthrough COVID-19 infections were increased post-COVID-19-vaccination among patients with various AIRD using a nationally sampled cohort.
Methods: We used U.S. National COVID Cohort Collaborative (N3C), the largest U.S. cohort of COVID-19 cases and controls that started in January 2020. We identified diagnoses of autoimmune or inflammatory rheumatic diseases based on one or more International Classification of Diseases (ICD)-10 codes for the following conditions through September 23, 2021: Rheumatoid Arthritis (RA); Spondyloarthritis (SpA); Gout; Systemic Lupus Erythematosus (SLE); Systemic Sclerosis (SSc); Polymyositis; Polymyalgia rheumatica (PMR); rheumatoid lung; the comparator group was people without rheumatic conditions (without-AIRD; no-rheumatic disease [RD]). Our primary outcome was breakthrough COVID-19 infections 14 days or later after full or partial COVID-19 vaccination (full vaccination defined as 2 doses of an mRNA vaccine or one dose of Janssen vaccine), as well as by the manufacturer of vaccine (i.e., Pfizer, Moderna, or Janssen). We performed unadjusted and multivariable-adjusted logistic regression analyses adjusted for age, gender, race/ethnicity, Quan-Charlson Comorbidity Index, and data partner to examine the odds of breakthrough COVID-19 infection compared to the absence of each condition.
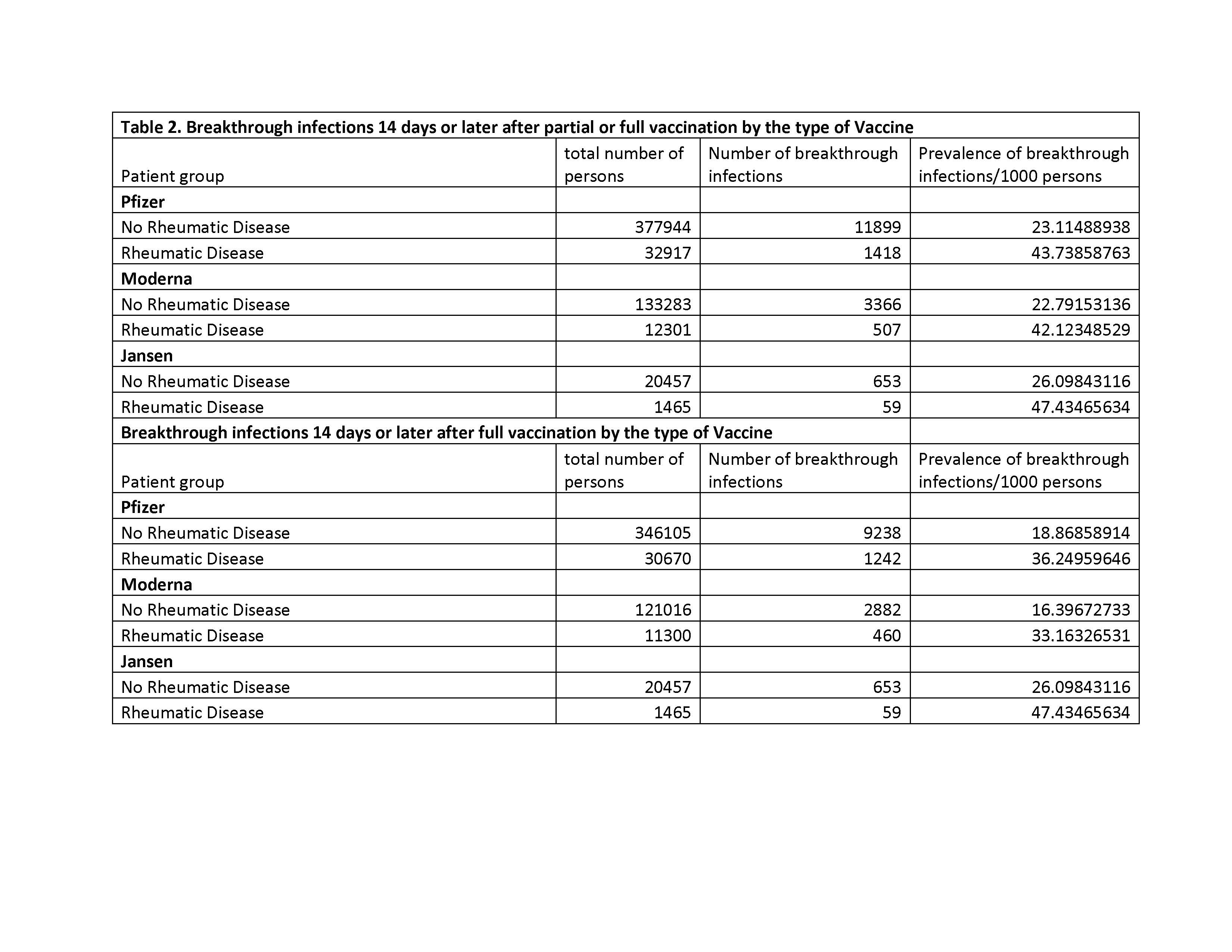
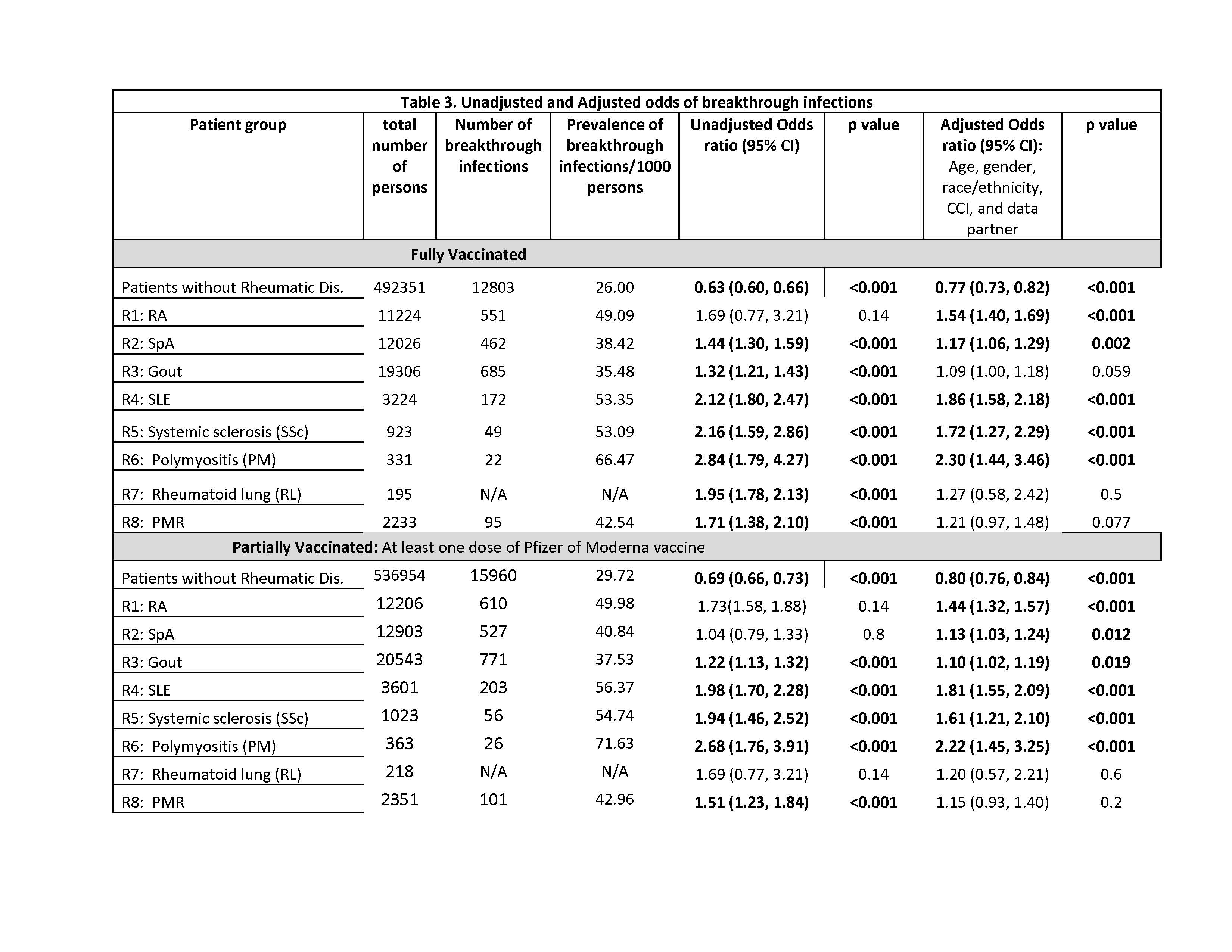
Results: A total of 584,257 patients met our eligibility criteria, of which 536,954 were people without AIRD (no-RD) and 47,303 had at least one of the AIRDs. Median (IQR) age was 50 and 66 years and 57% and 52% were female for no-RD and AIRD patients, respectively (Table 1). Prevalence of breakthrough COVID-19 infections/1,000 persons after full vaccination were as follows for without-AIRD vs. AIRD respectively: (1) Pfizer: 19 vs. 36; (2) Moderna, 16 vs. 33; and (3) Janssen, 26 vs. 47 (Table 2). The prevalence for partial vaccination followed similar patterns but were higher overall as compared to full vaccination. In those fully vaccinated, compared to people without each condition, the adjusted odds of breakthrough COVID-19 infections were increased significantly for RA, SpA, SLE, SSc, Polymyositis, but not for gout, PMR, or rheumatoid lung (Table 3): OR ranged from 1.2 (95% CI 1.1, 1.3) for SpA to 2.3 (95% CI 1.4, 3.5) for polymyositis. A similar pattern was seen after partial vaccination.
Conclusion: Patients with AIRDs appear to have an increased odds of breakthrough COVID-19 infections compared to patients without rheumatic disease. Further, the breakthrough COVID-19 infection risk varies by the type of AIRD. We will next examine the contribution of various drug exposures potentially influencing the higher risk of breakthrough infection among those with AIRDs. These results support the recent recommendations for COVID-19 booster in people with AIRD.
To cite this abstract in AMA style:
Singh J, Singh N, Anzalone A, Olex A, Sun J, Madhira V, Patel R. Breakthrough COVID-19 Infections Post-vaccination Among Immunocompromised Patients with Autoimmune or Inflammatory Rheumatic Diseases: A Retrospective Cohort Analysis from a U.S. Nationally-sampled Electronic Medical Record Data Repository [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/breakthrough-covid-19-infections-post-vaccination-among-immunocompromised-patients-with-autoimmune-or-inflammatory-rheumatic-diseases-a-retrospective-cohort-analysis-from-a-u-s-nationally-sampled-el/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/breakthrough-covid-19-infections-post-vaccination-among-immunocompromised-patients-with-autoimmune-or-inflammatory-rheumatic-diseases-a-retrospective-cohort-analysis-from-a-u-s-nationally-sampled-el/