Session Information
Date: Monday, October 27, 2025
Title: (1088–1122) Immunological Complications of Medical Therapy Poster
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: The cardiovascular risks associated with osteoporosis medications remain incompletely understood. Previous studies suggest that romosozumab (ROM) is most strongly associated with major adverse cardiovascular events (MACE), while denosumab (DMB) and teriparatide (TPT) have shown associations with select cardiovascular events in some reports [1–3]. Evidence for alendronate (ALN) and zoledronic acid (ZOL) is mixed, with some studies linking ZOL to atrial fibrillation (A-fib) [4–5]. Limited data exist for risedronate (RIS) and abaloparatide (ABA), with no consistent evidence of increased cardiovascular risk [1,4]. This study aims to evaluate the association between osteoporosis treatments and MACE.
Methods: We conducted a retrospective pharmacovigilance analysis using the FDA Adverse Event Reporting System (FAERS) from 2019 to 2024. All included drugs were FDA-approved by 2019. We examined reports of MACE—including myocardial infarction (MI), stroke, A-fib, and cardiac failure (CF)—associated with ALN, RIS, ZOL, DMB, TPT, ABA, and ROM. Reports involving individuals under 18 years of age were excluded. Disproportionality signal analysis using Reporting Odds Ratios (RORs) was conducted to evaluate MACE events associated with osteoporosis medications, comparing event reporting for each drug to the overall FAERS database. When the lower limit of the 95% Confidence Interval was >1, the ROR was deemed significant.
Results: We analyzed the characteristics of MACE reported in association with each osteoporosis medication. Stroke and CF were most frequent in the 65–85 age group across most drugs, while MI and A-fib were more common in those aged 18–64. Women were more frequently affected overall, except for A-fib, where men predominated.For MI, ROM had the highest ROR (2.38; 95% CI: 2.05–2.70; P < 0.0001), while DMB and ABA were linked to the lowest RORs. For stroke, ROM again showed the highest ROR (3.72), followed by ZOL (2.42). Only ABA demonstrated a negative association. For A-fib, ZOL had the highest ROR (3.22), followed by ROM and ALN; DMB showed a modest association (ROR: 1.20) relative to the other drugs. For CF, ROM had the highest ROR (3.76), followed by ALN (2.52) and RIS (1.86). ABA, TPT, and DMB were associated with the lowest RORs in this category.
Conclusion: We found that among the drugs considered, only ROM was associated with increased ROR for all MACE outcomes. In contrast, DMB and ABA demonstrated relatively low cardiovascular risk signals across most outcomes. ZOL showed notable positive associations with both stroke and A-fib, while ALN was linked to increased reporting of A-fib and CF.Our findings suggest that a patient’s individual risk for MACE should be considered when choosing osteoporosis drugs. Despite MACE risks, treating osteoporosis remains crucial, as fracture prevention significantly reduces morbidity and mortality. Limitations of our analysis include potential reporting bias, residual confounding, and its observational design, which precludes causal inference. Further prospective cohort studies and randomized clinical trials are needed to validate these associations and clarify the mechanisms of risk.
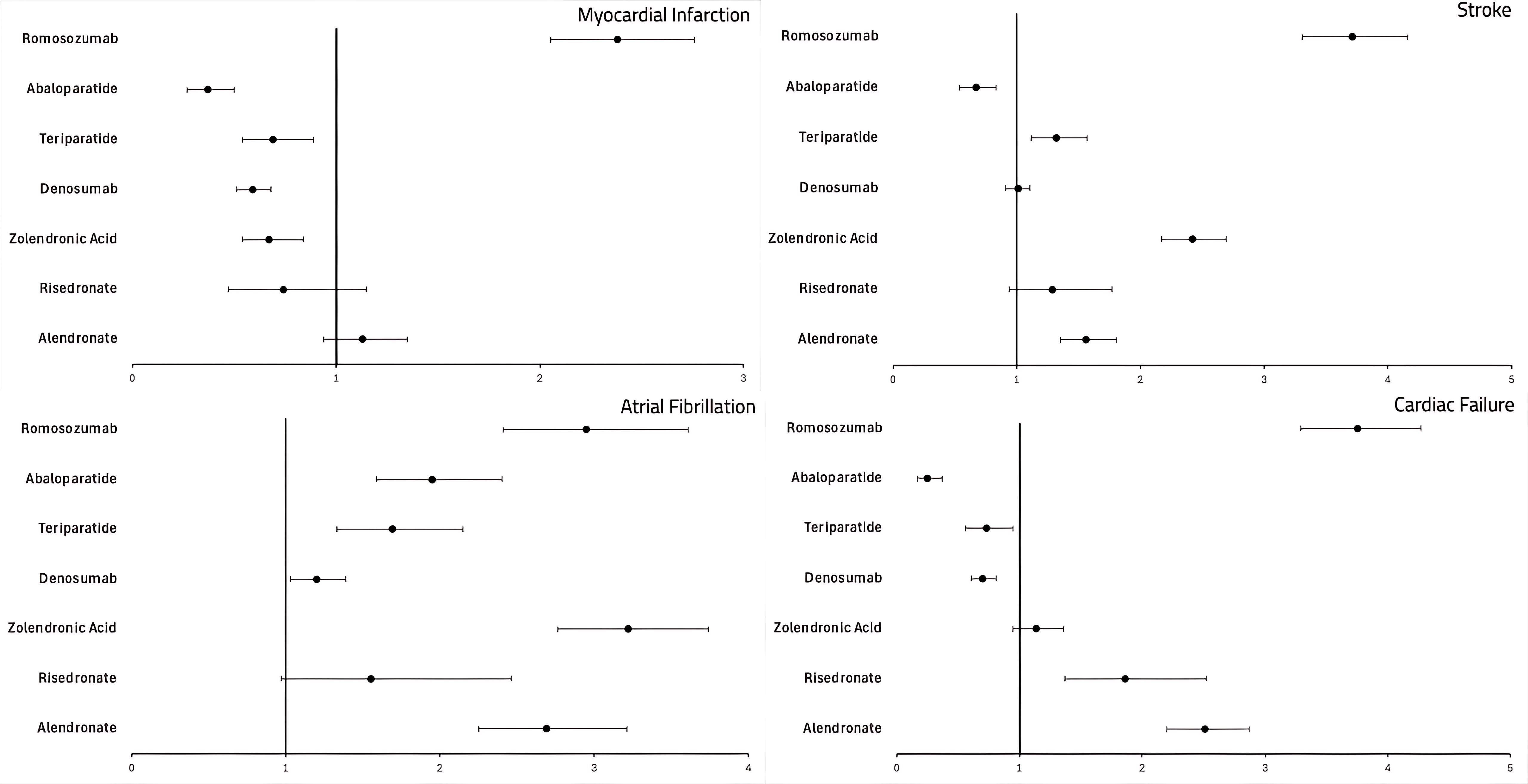 Table depicting the calculated RORs of MACE associated with the medications used for the treatment of osteoporosis.
Table depicting the calculated RORs of MACE associated with the medications used for the treatment of osteoporosis.
.jpg) Forest plot depicting the association of MACE with the medications used for the treatment of osteoporosis.
Forest plot depicting the association of MACE with the medications used for the treatment of osteoporosis.
To cite this abstract in AMA style:
Sondhi M, Singh N, Carkin J, Hughes G. Breaking Bones, Breaking Hearts: A FAERS Perspective on Osteoporosis Medications [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/breaking-bones-breaking-hearts-a-faers-perspective-on-osteoporosis-medications/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/breaking-bones-breaking-hearts-a-faers-perspective-on-osteoporosis-medications/
