Session Information
Date: Saturday, November 6, 2021
Title: Abstracts: Osteoporosis & Metabolic Bone Disease – Basic & Clinical Science (0445–0448)
Session Type: Abstract Session
Session Time: 10:00AM-10:15AM
Background/Purpose: Orally-available bone anabolic agents represent a major unmet medical need for patients with osteoporosis. Widespread use of parathyroid hormone (PTH)-based osteo-anabolic therapies is limited by the need for daily injections. PTH stimulates bone formation via activating a signaling cascade in osteocytes that inhibits salt inducible kinases (SIK) 2 and 3. The purpose of this study is to test a novel, orally-available SIK2/SIK3 inhibitor (SIK2/SIK3i) in preclinical osteoporosis models.
Methods: An osteocyte-like cell line was treated with PTH and SIK2/SIK3i. Cells were treated for 60 minutes followed by immunoblotting for phosphorylated forms of SIK2/SIK3 substrates HDAC4/5. Cells were treated for 4 hours followed RT-qPCR to measure gene expression.
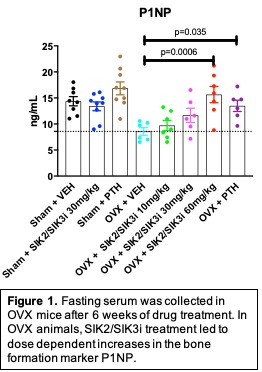
A SIK2/SIK3 inhibitor was tested in two models of post-menopausal bone loss: oophorectomized (OVX) mice and rats. Direct comparison was made to treatment with intermittent PTH. 64 twelve-week-old Balb/c mice were subjected to sham (n=26) or OVX (n=38) surgery. 5 weeks later, mice were treated for 6 weeks with vehicle (n=15), SIK2/SIK3i 10 mg/kg PO BID (n=8), SIK2/SIK3i 30 mg/kg PO BID (n=17), SIK2/SIK3i 60 mg/kg PO BID (n=8), or PTH 1-34 100 mcg/kg SC (n=16). Upon sacrifice, fasting serum was collected to measure bone turnover markers P1NP and CTX, along with femur and L5 vertebrae for µCT, and femurs for histomorphometry.
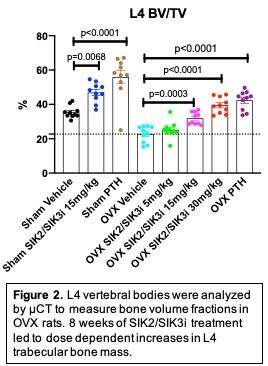
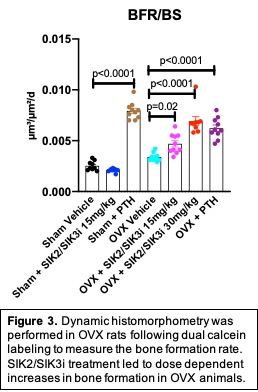
Next, 80 twelve-week-old Sprague-Dawley rats were subjected to sham (n=30) or OVX (n=50) surgery. 8 weeks later, rats were treated for 8 weeks with vehicle (n=20), SIK2/SIK3i 5 mg/kg PO BID (n=10), SIK2/SIK3i 15 mg/kg PO BID (n=20), SIK2/SIK3i 30 mg/kg PO BID (n=10), and PTH 1-34 40 mcg/kg SC (n=20). Fasting serum was collected at 1, 4, and 8 weeks. At sacrifice, femur and L4 vertebral bodies were collected for µCT, histomorphometry, and biomechanical testing. Two-way ANOVA followed by Tukey post-hoc tests were performed.
Results: In Ocy454 cells, SIK2/SIK3i treatment led to dose-dependent reductions in SIK2/SIK3 substrate HDAC4/5 phosphorylation. SIK2/SIK3i treatment suppressed SOST mRNA expression (EC50 = 310 nM).
In OVX mice, SIK2/SIK3i led to dose-dependent increases in the bone formation marker P1NP (p=0.0006) (Figure 1). In OVX mice, SIK2/SIK3i increased L5 bone mass (p=0.0001) and femur cortical thickness (p=0.038) by µCT. Histomorphometry showed that SIK2/SIK3i increased mineralizing surface (p=0.0142) and bone formation rate (p=0.009).
Increased bone anabolism was also observed in OVX rats treated with SIK2/SIK3i, which also led to initial increases in serum P1NP, followed by increased levels of both P1NP and CTX at later time points. SIK2/SIK3i increased trabecular bone mass in OVX rats in femur (p< 0.001) and L4 vertebrae (p< 0.001) (Figure 2). Mechanical testing of L4 vertebrae demonstrated proportionate increases in bone strength and bone mass. Finally, SIK2/SIK3i increased bone formation rate in a dose-dependent manner (p< 0.001) to a degree similar to that of PTH (Figure 3).
Conclusion: SIK2/SIK3i increases trabecular bone formation and bone mass in OVX rodents. Orally-available small molecule SIK2/SIK3 inhibitors may represent a promising new treatment strategy for post-menopausal osteoporosis.
To cite this abstract in AMA style:
Tang C, Verma S, De Vos S, Amantini D, Clement-Lacroix P, Desroy N, Speziale A, Brooks D, Bouxsein M, da Silva Martins J, Zhao Y, Kronenberg H, Wein M. Bone Anabolic Effects of a Novel Orally-Available Small Molecule SIK2/SIK3 Inhibitor [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/bone-anabolic-effects-of-a-novel-orally-available-small-molecule-sik2-sik3-inhibitor/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/bone-anabolic-effects-of-a-novel-orally-available-small-molecule-sik2-sik3-inhibitor/