Session Information
Date: Sunday, November 7, 2021
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: ReFLECT study has been carried out to investigate real life use of CT-P13, the first monoclonal antibody biosimilar to infliximab (IFX) originator.
Methods: ReFLECT is a multicentre, prospective, observational study conducted in France to determine the characteristics of patients (pts) receiving CT-P13, its effectiveness and safety in a real-life setting. Eligible pts were both IFX naive pts (IFX-N) starting CT-P13 and those who have been switched from infliximab originator (IFX-S) to CT-P13. Interim results in adult pts with rheumatic diseases using descriptive statistical analyses from inclusion to a 24-month-follow-up period are presented here.
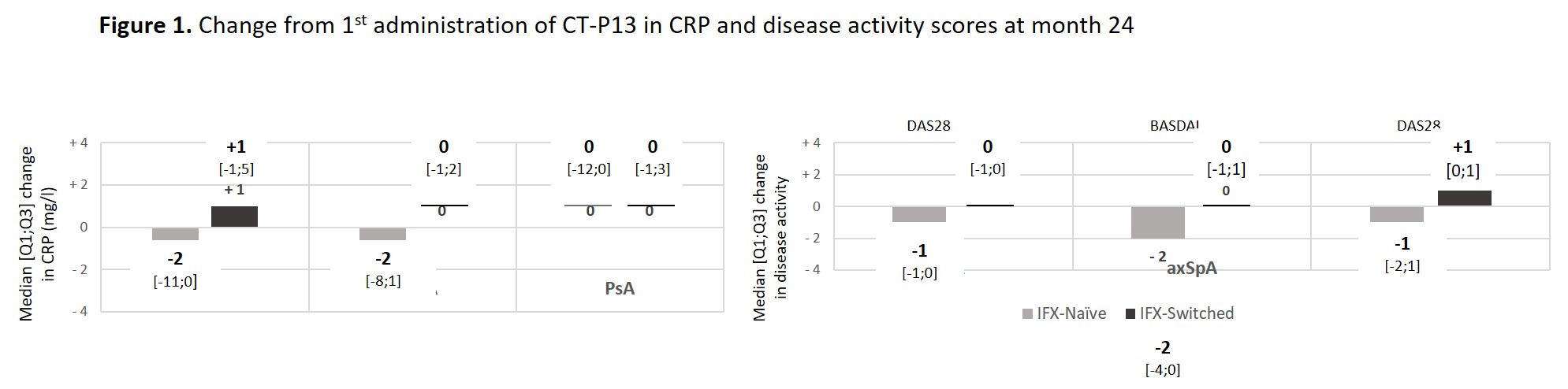
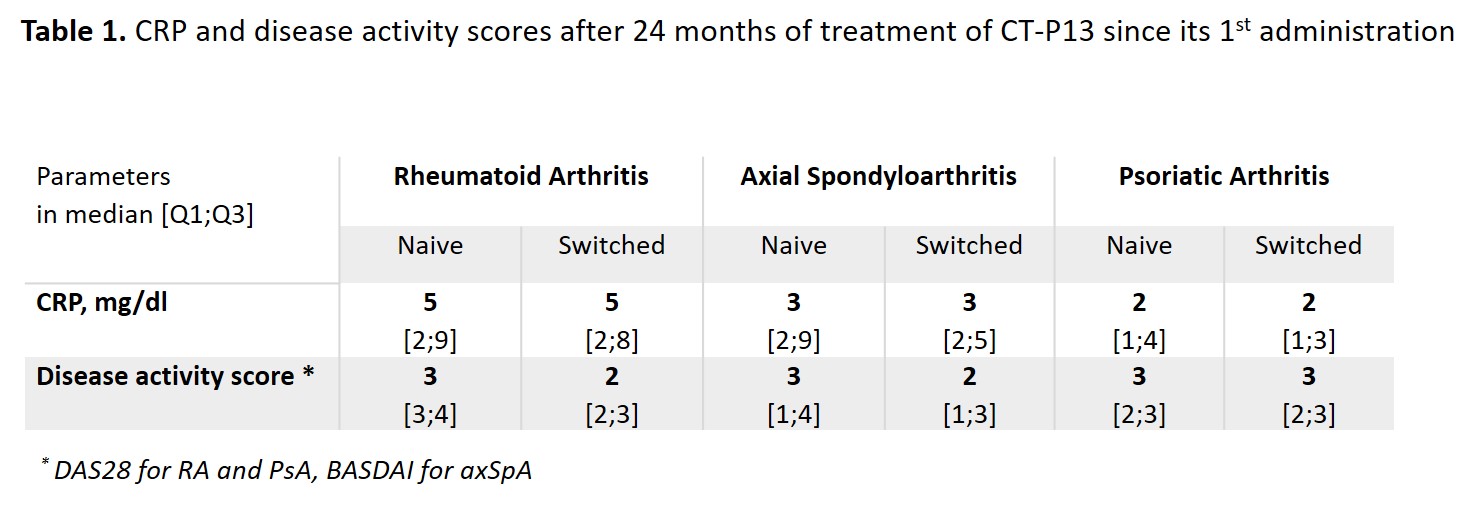
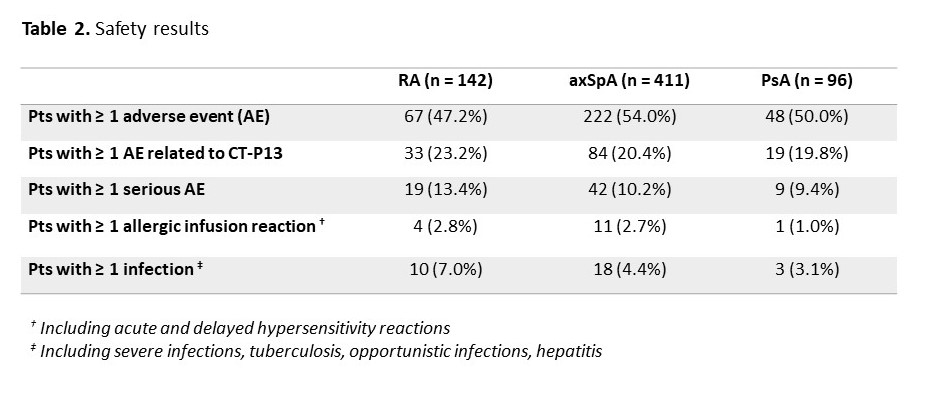
Results: Among the 1370 adult pts included between October 2016 and April 2019, data were analysed for 142 pts with rheumatoid arthritis (RA; 23.9% males; mean age: 61.5±10.9 years; 80 IFX-N / 61 IFX-S), 411 with axial spondyloarthritis (axSpA; 58.9% males; 48.1±13.1 years; 228 IFX-N / 179 IFX-S), and 96 with psoriatic arthritis (PsA; 41.7% males; 53.4±14.1 years; 55 IFX-N / 40 IFX-S) after a median duration between 8.7 and 12.9 years since diagnosis. At inclusion, 67.9%, 24.1% and 50% had a concomitant treatment with methotrexate. At the time of the first administration of CT-P13, disease had been active in 94.2%, 76.6% and 83.3% of the IFX-N pts compared with 45.3%, 24.0% and 32.0% of the IFX-S pts respectively. From the first administration of CT-P13 to month 24, both CRP levels and disease activity remained stable in the IFX-S pts and as expected, improvement was observed in the IFX-N pts (Figure 1). In naive pts, after 24 months of treatment since its first administration, CT-P13 brought both CRP and disease activity down to levels comparable to those seen in pts having switched from the IFX originator to CT-P13 (Table 1). After 24 months of follow-up, 70 to 80% of pts in each group, whether IFX-N or IFX-S, remained on treatment with CT-P13, RA naive pts aside (53.8%). Main reason for CT-P13 withdrawing was treatment failure (including primary non-response and secondary loss of response) in both naive and switched pts: 31.6% and 13.1% of RA, 18.1% and 12.8% of axSpA, 9.1% and 15.8% of PsA pts respectively. Withdrawing for intolerance involved 6.3% and 1.6% of RA, 2.2% and 1.1% of axSpA, 1.8% and 2.6% of PsA in naive and switched pts respectively. Safety results are reported in Table 2.
Conclusion: Year 2 follow-up data indicate that CT-P13 effectively induced improvement in disease activity in pts with RA, axSpA and PsA receiving infliximab for the first time and maintained stable disease activity in pts having switched from IFX originator to CT-P13. This real-life study did not highlight any new safety concerns.
To cite this abstract in AMA style:
MAROTTE H, Assing M, Mammar N, Brault Y, Fautrel B. Biosimilar Infliximab Therapy in Rheumatoid Arthritis, Axial Spondyloarthritis and Psoriatic Arthritis: A Long-term Follow-up Study on Infliximab-naive Patients and Switched Patients from the Originator to the Biosimilar CT-P13 [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/biosimilar-infliximab-therapy-in-rheumatoid-arthritis-axial-spondyloarthritis-and-psoriatic-arthritis-a-long-term-follow-up-study-on-infliximab-naive-patients-and-switched-patients-from-the-originat/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/biosimilar-infliximab-therapy-in-rheumatoid-arthritis-axial-spondyloarthritis-and-psoriatic-arthritis-a-long-term-follow-up-study-on-infliximab-naive-patients-and-switched-patients-from-the-originat/