Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Behçet’s disease (BD) is a variable vessel vasculitis and typically presents with mucocutaneous involvement. However, any organ can be affected, being the neurological affectation (neurobehçet, NB) one of the most serious manifestations.
Our aim was to assess the efficacy and safety of biological therapy as treatment of NB.
Methods: We set up a multicenter observational study of 31 patients with NB on treatment with biological therapy (BT). NB diagnosis was made by neuroimaging, CSF analysis and/or suggestive clinical signs of central and/or peripheral nervous system involvement, excluding infectious causes or more prevalent pathology. Results are expressed as mean±SD or as median and interquartile range (IQR) as appropriate.
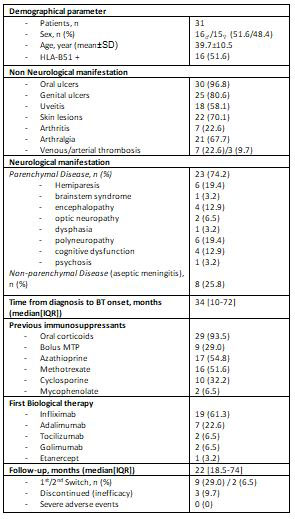
Results: 31 patients (16♂ / 15♀) with an average age of 39.7 ± 10.5 years. HLA-B51 was positive in 51.6% of the patients. Table shows the non-neurological manifestations. Regarding the neurological manifestations, 23 patients (74.2%) had parenchymal involvement (hemiparesis (n=6), brainstem involvement (n=1), encephalopathy (n = 4), optic neuropathy (n=3), dysphasia (n=1), polyneuropathy (n=6), cognitive impairment (n=4), and non-steroidal psychosis (n=1), while the remaining 8 patients (25.8%) presented aseptic meningitis as a non-parenchymal affectation (Table).
Prior to BT, patients had received the following treatment: oral prednisone (n=29), methylprednisolone bolus (n=9), CsA (n=10), AZA (n=17), MTX (n=16) and mycophenolate (n=2).
After a median of 34 [10-72] months since the beginning of the neurological symptoms, the following BT was initiated: infliximab (IFX)(n=19), adalimumab (ADA)(n=7), tocilizumab (TCZ) (n=2), golimumab (GOL) (n=2) and Etanercept (ETN) (n=1). A first switch to ADA was necessary in 9 patients with IFX due to primary failure. In addition, 2 of them needed a second switch to TCZ, getting a partial response. The BT was discontinued in 5 patients, 2 of them for obtaining clinical remission and the remaining 3 for inefficacy.
After a median follow-up of 5.4±4.6 years, complete response was obtained in 15 patients, partial response in 11 and no response in the remaining 3. We observed an anaphylactic reaction and psoriasis induced by IFX, without other serious adverse events (Table).
Conclusion: BT, especially anti-TNF, seems effective and safe for treatment in NB.
To cite this abstract in AMA style:
Gonzalez-Mazon I, Sanchez-Bilbao L, Martín-Varillas J, Atienza-Mateo B, Calvo Río V, Castañeda S, Vicente-Rabaneda E, Maiz O, blanco a, Moriano C, Díez E, Andreu J, Delgado-Beltrán C, Loredo-Martinez M, Narváez J, Ramos-Calvo A, Sivera F, Raya E, Ortego Centeno N, callejas rubio J, Brandy-Garcia A, Olivé-Marqués A, Fernández S, Gómez De La Torre R, Torre-Salaberri I, Sanchez J, Urruticoechea-Arana A, salgado-Pérez E, Melero R, Martinez O, Romero-Yuste S, Gonzalez-Gay M, Blanco R. Biological Therapy in Neurobehçet: Multicenter Study of 31 Patients [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/biological-therapy-in-neurobehcet-multicenter-study-of-31-patients/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/biological-therapy-in-neurobehcet-multicenter-study-of-31-patients/