Session Information
Date: Tuesday, October 28, 2025
Title: (2338–2376) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Previous research has shown that women with psoriatic arthritis (PsA) often have lower rates of clinical response to medication but less severe radiographic joint damage. Despite this, few randomized controlled trials (RCTs) in PsA report sex-disaggregated results. Here, we assessed sex-disaggregated radiographic progression (RP) in DISCOVER-2 (NCT03158285), a Phase 3 study of biologic-naïve PsA pts treated with GUS, considering sex differences in structural damage and whether this is associated with early improvements in joint disease activity (DA).
Methods: Biologic-naïve pts with active PsA were randomized (1:1:1) to GUS 100 mg every 4 weeks (Q4W); GUS 100 mg at W0, W4, then Q8W; or placebo with crossover to GUS 100 mg Q4W at W24. Early (W8) response in joint DA, (based on clinical DA Index for PsA [cDAPSA LDA; ≤13]), was assessed among pts with cDAPSA >13 at BL, without adjustment for sex-specific differences at BL. Multivariate repeated measures mixed models assessed associations between sex and changes in total PsA-modified van der Heijde-Sharp [vdH-S] score through W100 (Fig 1), association between early response in joint DA and RP when stratified by sex (Fig 2); time-averaged and visit-specific LSM changes.
Results: Of 739 PsA pts enrolled, 47.5% were female. At BL, female vs male pts were more likely to be older (29.3% vs 21.9% ≥55 years of age; p=0.0204) and have dactylitis (60.6% vs 50.3%; p=0.0049), and on average had higher BMI (29.5 vs 28.4 kg/m2; p=0.0144), lower CRP levels (1.6 vs 2.3; p< 0.0001), less extensive/severe psoriasis (Psoriasis Area and Severity Index [PASI] score: 7.5 vs 12.1; p< 0.0001), more functional disability (Health Assessment Questionnaire-Disability Index [HAQ-DI] score: 1.4 vs 1.2; p< 0.0001), and worse fatigue (Functional Assessment of Chronic Illness Therapy [FACIT]-Fatigue score: 28.5 vs 30.9; p=0.0007). BL cDAPSA (46.1 vs 46.4) and PsA-modified vdH-S (26.2 vs 25.5) scores were similar (p >0.05) between sexes.In unadjusted analyses of GUS-treated pts, male sex was associated with greater progression of structural damage through W100 (ΔLSM=1.02; p=0.0260). After adjusting for known risk factors of RP, BL characteristics with sex-specific differences in the pooled cohort, and disease duration and non-biologic DMARD use at BL, the difference between sexes in RP decreased but remained significant (ΔLSM=0.90; p=0.0406); LSM changes from BL in PsA-modified vdH-S scores in males vs females were 1.36 vs 0.69 (p=0.0502) at W52 and 2.22 vs 1.10 (p=0.0475) at W100 (Fig 1). Early (W8) cDAPSA LDA was achieved by 18.9% of men vs 15.3% of women. In men, this was associated with significantly less RP through W100 (time-averaged change in PsA-modifed vdH-S scores from BL to W100 in cDAPSA LDA responders vs nonresponders: 0.39 vs 2.24; p=0.0288); in females only, numerical differences were observed (-0.15 vs 0.91; p=0.1732; Fig 2).
Conclusion: These results confirm the independent association of male sex with more rapid RP. The previously reported relationship between early improvement in joint DA and diminished RP (Mease PJ, et al. Clin Rheumatol 2024) was found to be stronger in males than females, which may be due to the lower rate of RP in females.
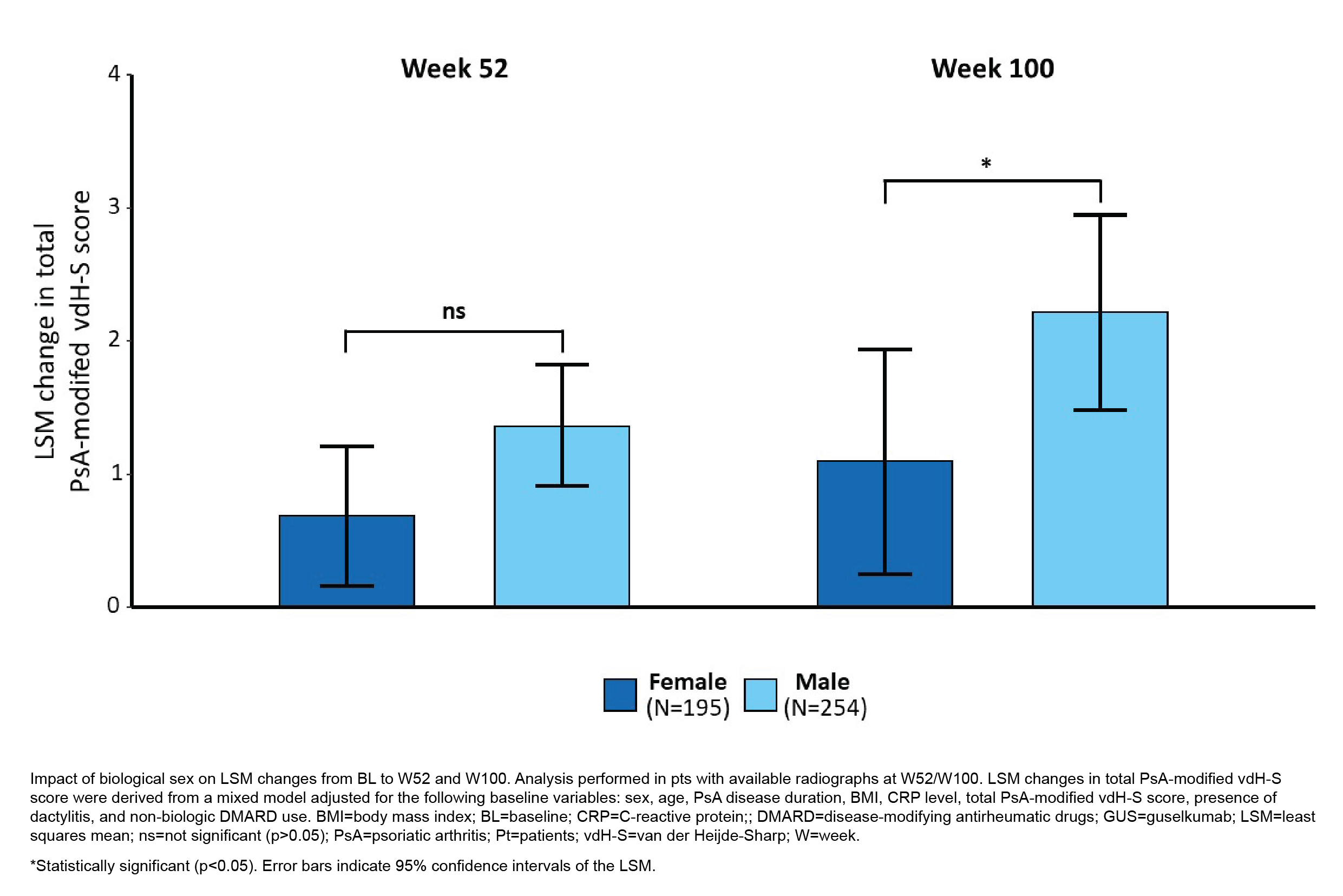 Figure 1. Change in Total PsA-modified vdH-S Score from BL Through W100 in Male and Female PsA Pts Treated with GUS
Figure 1. Change in Total PsA-modified vdH-S Score from BL Through W100 in Male and Female PsA Pts Treated with GUS
.jpg) Figure 2. Multivariate Association of cDAPSA LDA Achievement at W8 with Time-Averaged Change in Total PsA-modified vdH-S Score from BL Through W100 in Male and Female PsA Pts Treated with GUS
Figure 2. Multivariate Association of cDAPSA LDA Achievement at W8 with Time-Averaged Change in Total PsA-modified vdH-S Score from BL Through W100 in Male and Female PsA Pts Treated with GUS
To cite this abstract in AMA style:
Gladman D, Eder L, Selmi C, Mease P, Ogdie A, Lozenski K, Sharaf M, Rampakakis E, Vegas L, Coates L. Biological Sex-Related Differences in Radiographic Progression and Relationship with Early Clinical Response: Post Hoc Analysis of a Phase 3, Randomized, Double-Blind, Placebo‑Controlled Study in Biologic-Naive Participants with Active Psoriatic Arthritis Treated with Guselkumab [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/biological-sex-related-differences-in-radiographic-progression-and-relationship-with-early-clinical-response-post-hoc-analysis-of-a-phase-3-randomized-double-blind-placebo%e2%80%91controlled-study/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/biological-sex-related-differences-in-radiographic-progression-and-relationship-with-early-clinical-response-post-hoc-analysis-of-a-phase-3-randomized-double-blind-placebo%e2%80%91controlled-study/
