Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
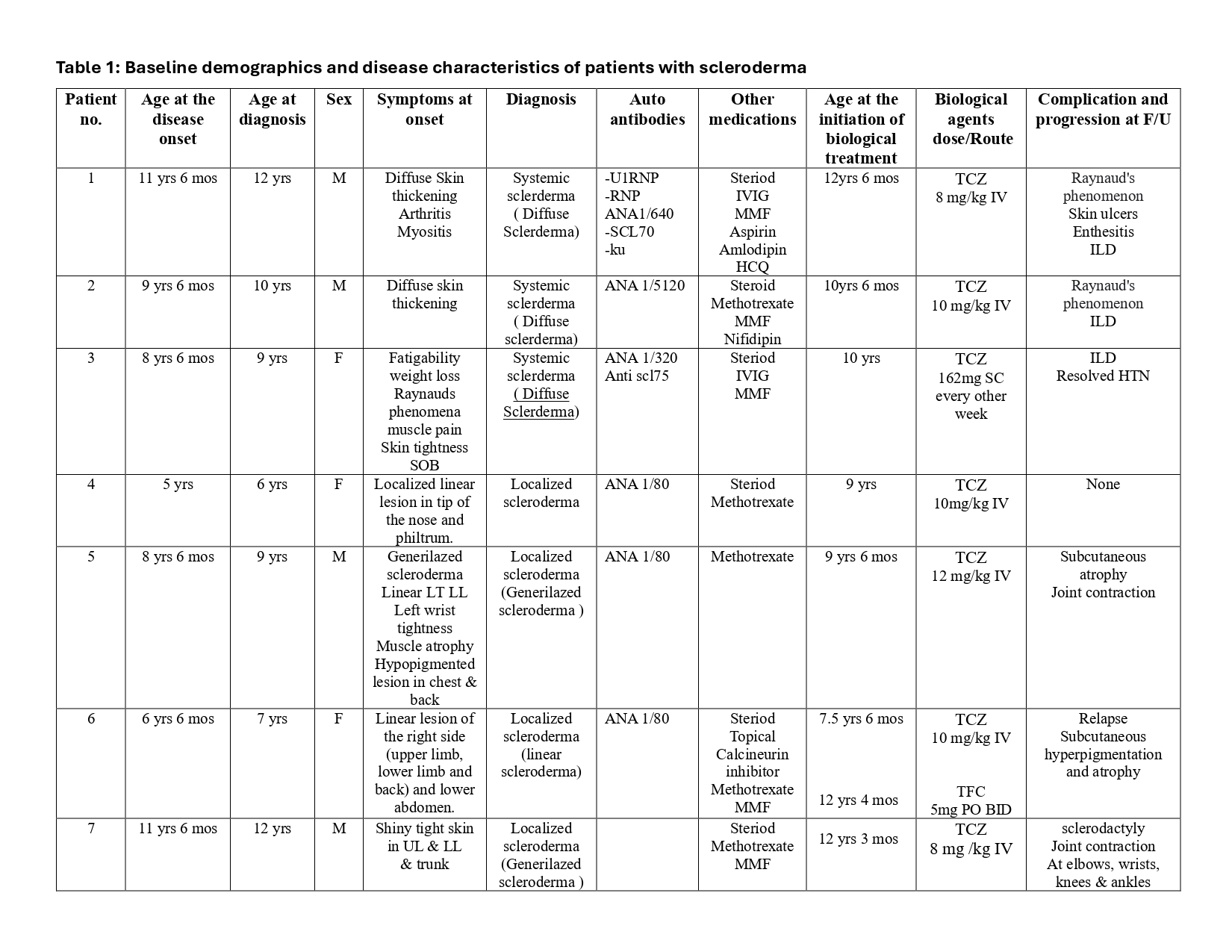
Background/Purpose: Scleroderma is autoimmune disease that may result in changes to the skin, blood vessels, muscles, and internal organs. It can be either Localized Scleroderma (LS) or Systemic Sclerosis (SSc). Early diagnosis, proper assessment and effective treatment are crucial to improve the long-term outcome.Tocilizumab, an IL-6 receptor antagonist, and tofacitinib, a JAK inhibitor, have shown promising results in small series and case reports, but pediatric data remain limited.The current analysis was conducted from a single center in Saudi Arabia to investigate the long-term efficacy and safety of tocilizumab (TCZ) and tofacitinib (TFC) in patients with SSc and LS.
Methods: We conducted a retrospective chart review of pediatric patients diagnosed with LS or SSc who received TCZ or TFC in a single center at Prince Sultan Military Medical City in Saudi Arabia. Overall, seven patients were included in the study (3 Females and 4 Males with a mean age of 9 years (range 6-12 years).The modified Rodnan skin score (mRSS), carbon-monoxide diffusion capacity (DLCO), thorax high-resolution tomography (HRCT), patient global assessment (PGA), and Juvenile Systemic Sclerosis Severity (J4S) score were used in patients with SSc as endpoints.The Localized Scleroderma Cutaneous Assessment Tool (LoSCAT) and physician global assessment of activity and damage (PGA-A and PGA-D) were used in patients with localized scleroderma as endpoints to evaluate TCZ and TFC treatment response at 12 months post-treatment.
Results: Seven children with scleroderma were treated with TCZ, in addition to conventional treatment. The median age at the disease onset is 8.5 years (5–11.5) and at the diagnosis is 9 years (6-12).Three JSS patients with interstitial lung disease were treated with TCZ with a median treatment duration of 19 (14-24) months. All three patients had radiologically confirmed improvement in thorax HRCT at 12 months post-treatment. They had decreased PGA (mean pre-treatment PGA 5 vs. 2.6 post-treatment PGA at 12 months). They had increased DLCO (mean pre-treatment DLCO 47% vs.12 months post-treatment DLCO 72.6% after the TCZ treatment). In all patients, mRSS and the J4S decreased: 33.3 vs. 14.3 and 10 vs. 4.3, respectively. Three LS patients were treated with TCZ with a median treatment duration of 22 (6 -48) months. They had decreased PGA-A and PGA-D, with mean pre-treatment PGA-A 7 vs 1.7 in 12 months post-treatment, while the mean pre-treatment PGA-D 6.5 vs 4 in 12 months post-treatment. They had also decreased in LoSSI and LoSDI: 25 vs 2.5 and 21.5 vs 14.7, respectively. One patient with refractory LS was escalated to TFC (5 mg BID) after a partial response to TCZ. She had decreased PGA-A and PGA-D: pre-treatment 6 vs 1 and 5 vs 3 in 6 months post-treatment, respectively. She had also reduced LoSSI and LoSDI: 7 vs 1 and 7 vs 4, respectively. TCZ and TFC treatment are tolerated without side effects.
Conclusion: This case series highlights the promising role of Tocilizumab as a therapeutic option in managing refractory pediatric scleroderma in both LS and SSc. Tofacitinib demonstrated also additional benefit in controlling refractory Localized Scleroderma . Long-term studies with a larger number of patients are needed to provide more relevant data.
To cite this abstract in AMA style:
ALMARRI M, Alwakeel D, Alrowais A, Asiri A. Biologic Therapy for Refractory Juvenile Scleroderma: a retrospective case-series from a single tertiary care center in Saudi Arabia. [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/biologic-therapy-for-refractory-juvenile-scleroderma-a-retrospective-case-series-from-a-single-tertiary-care-center-in-saudi-arabia/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/biologic-therapy-for-refractory-juvenile-scleroderma-a-retrospective-case-series-from-a-single-tertiary-care-center-in-saudi-arabia/

.jpg)
.jpg)