Session Information
Date: Sunday, November 12, 2023
Title: (0380–0422) RA – Diagnosis, Manifestations, and Outcomes Poster I
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Although the modern RA treatment armamentarium has led to significant improvements in response rates and outcomes, some RA patients inadequately respond to multiple lines of treatment. The ability to study these “treatment refractory” RA patients is limited by small numbers of patients meeting criteria, even at high volume centers. In the cancer field, the general challenge of engaging small patient subsets for research led to the development of a novel “crowdsourced” bioinformatics research platform to recruit and study extreme outlier responses to chemotherapy from multiple sites/sources. Here we report the adaption of this novel platform to study genetic and clinical risk factors for inadequate response to multiple biologic and targeted synthetic DMARD medications (b/tsDMARDs) in RA.
Methods: RA Non-responders to Treatment (RANT) is a “crowdsourced” cohort study (target size n=300) using a bioinformatics platform investigating genetic and clinical predictors of non-response to b/tsDMARD medications. RA patients who have failed two or more b/tsDMARDs (including at least one TNF inhibitor) are eligible. A combination of traditional and multimedia recruitment advertises the study: direct in-person clinic recruitment, dissemination of trial information to patients identified in established RA registries (ACR RISE, FORWARD), and social media publicizing the study to patients and rheumatologists via Twitter and Facebook. Study details are posted for the general public on clinicaltrials.gov.
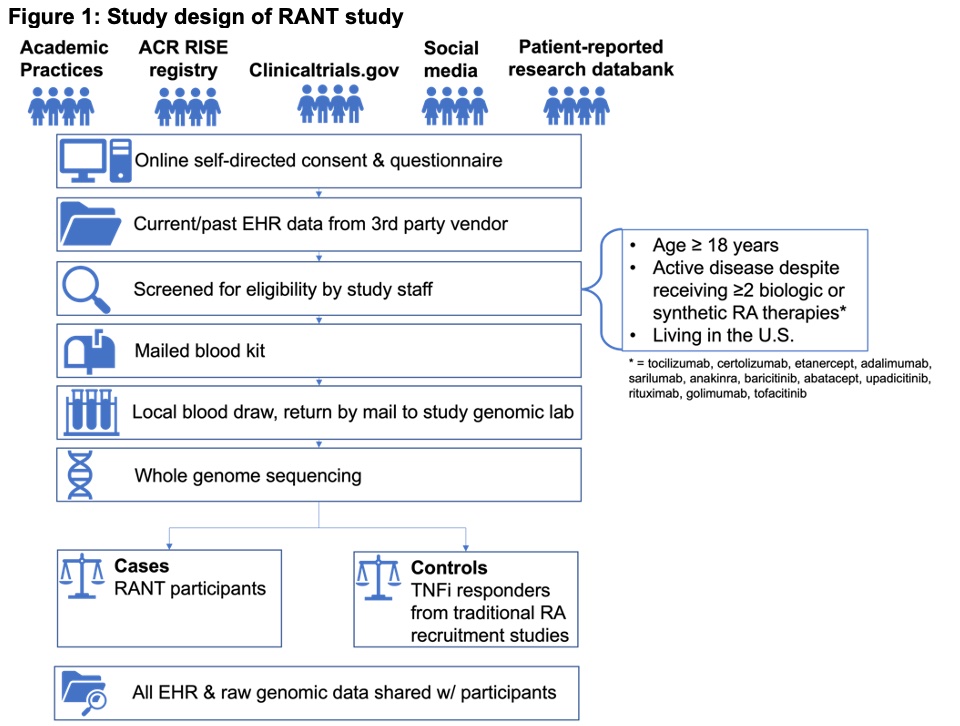
The study process is detailed in Figure 1. Interested patients complete a self-directed online informed consent that includes access to electronic health records though a third-party vendor as well as RA treatment and disease status questionnaires. After study staff confirm eligibility by reviewing patient responses, patients receive a blood sample kit by mail, have blood sample tubes drawn locally, and return the kit by mail to the study genomic lab for whole genome sequencing. Clinical and genetic factors of RA treatment non-responders will be compared to established groups of RA treatment responders from established research cohorts. Patients will receive a copy of their medical records and genetic sequence data.
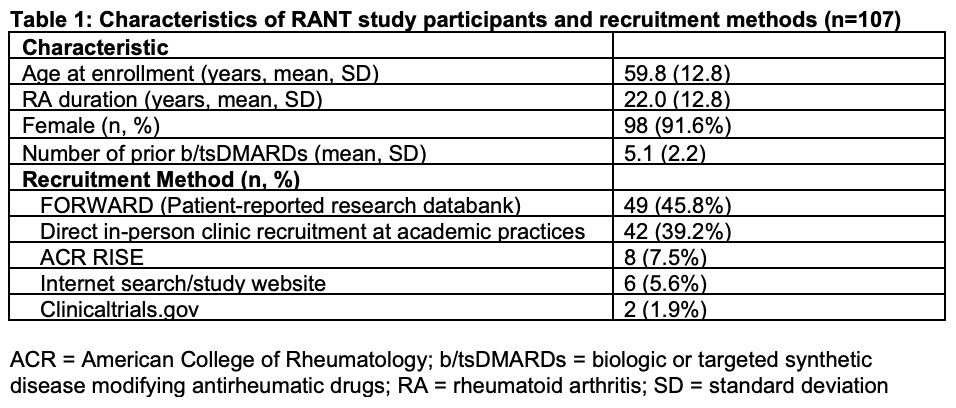
Results: Baseline characteristics of the first 107 recruited patients are detailed in Table 1. The majority were female (91.6%) with mean age 59.8 years and mean RA duration of 22 years. The mean number of b/tsDMARDs prescribed was 5.1 (SD 2.2). The most successful recruitment methods have been through an established patient-reported research databank (45.8%) and direct in-person recruitment of patients in clinic (39.2%).
Conclusion: This protocol describes a “crowdsourced” recruitment and online enrollment/consent procedures to investigate RA treatment non-responders. Similar methods may be useful for investigating rare rheumatology diseases, outcomes, or patient subsets that are difficult to reach or recruit by traditional research methods. Study results will demonstrate the feasibility of these research methods in future rheumatology research and inform the potential clinical and genetic predictors of treatment refractory RA.
To cite this abstract in AMA style:
McDermott G, Jeffway M, Seyok T, Zhang H, Dahal K, Weisenfeld D, Vella M, Johansson T, Schmajuk G, Michaud K, Perry C, Churchill S, Liao K. Bioinformatics Platform to Study the Genetics of Biologic DMARD Non-responders: Design and Protocol of the RA Non-responders to Treatment (RANT) Study [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/bioinformatics-platform-to-study-the-genetics-of-biologic-dmard-non-responders-design-and-protocol-of-the-ra-non-responders-to-treatment-rant-study/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/bioinformatics-platform-to-study-the-genetics-of-biologic-dmard-non-responders-design-and-protocol-of-the-ra-non-responders-to-treatment-rant-study/