Session Information
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: Patients with psoriatic arthritis (PsA) have identified fatigue and pain as important features relevant to their burden of disease.1 Bimekizumab (BKZ), a monoclonal IgG1 antibody that selectively inhibits IL-17F in addition to IL-17A, has demonstrated improvements in patient-reported symptoms up to 3 years in a phase 2b study.2 This analysis aimed to assess the impact of BKZ treatment vs placebo (PBO) on the clinically relevant symptoms of patient‑reported fatigue and pain in patients with active PsA who are biologic DMARD (bDMARD)-naïve or had inadequate response to 1–2 TNF inhibitors (TNFi-IR), using pooled data from BE OPTIMAL and BE COMPLETE.
Methods: BE OPTIMAL (NCT03895203) and BE COMPLETE (NCT03896581) are phase 3 trials assessing BKZ in patients with PsA who are bDMARD-naïve or TNFi-IR, respectively. Both trials had a 16-week (wk) double-blind and placebo‑controlled phase, allowing data to be pooled across the trials. Patients in BE OPTIMAL were randomized 3:2:1 to subcutaneous (sc) BKZ 160 mg every 4 wks (Q4W), PBO, or sc adalimumab (reference arm) 40 mg every 2 weeks (Q2W); and in BE COMPLETE 2:1 to sc BKZ 160 mg Q4W or PBO. We present pooled and individual study data to Wk 16 for BKZ and PBO treatment arms. Functional Assessment of Chronic Illness Therapy-Fatigue subscale Minimum Clinically Important Difference (FACIT-Fatigue MCID; ≥4-point improvement from baseline) and clinically important improvements (≥30/50/70%) in Patient’s Assessment of Arthritis Pain (PtAAP) are reported.3 Non-responder and multiple imputation (NRI, MI) were used for missing binary and continuous variables, respectively.
Results: A total of 1,073/1,112 (96.5%) patients randomized to BKZ or PBO completed Wk 16 in BE OPTIMAL and BE COMPLETE.
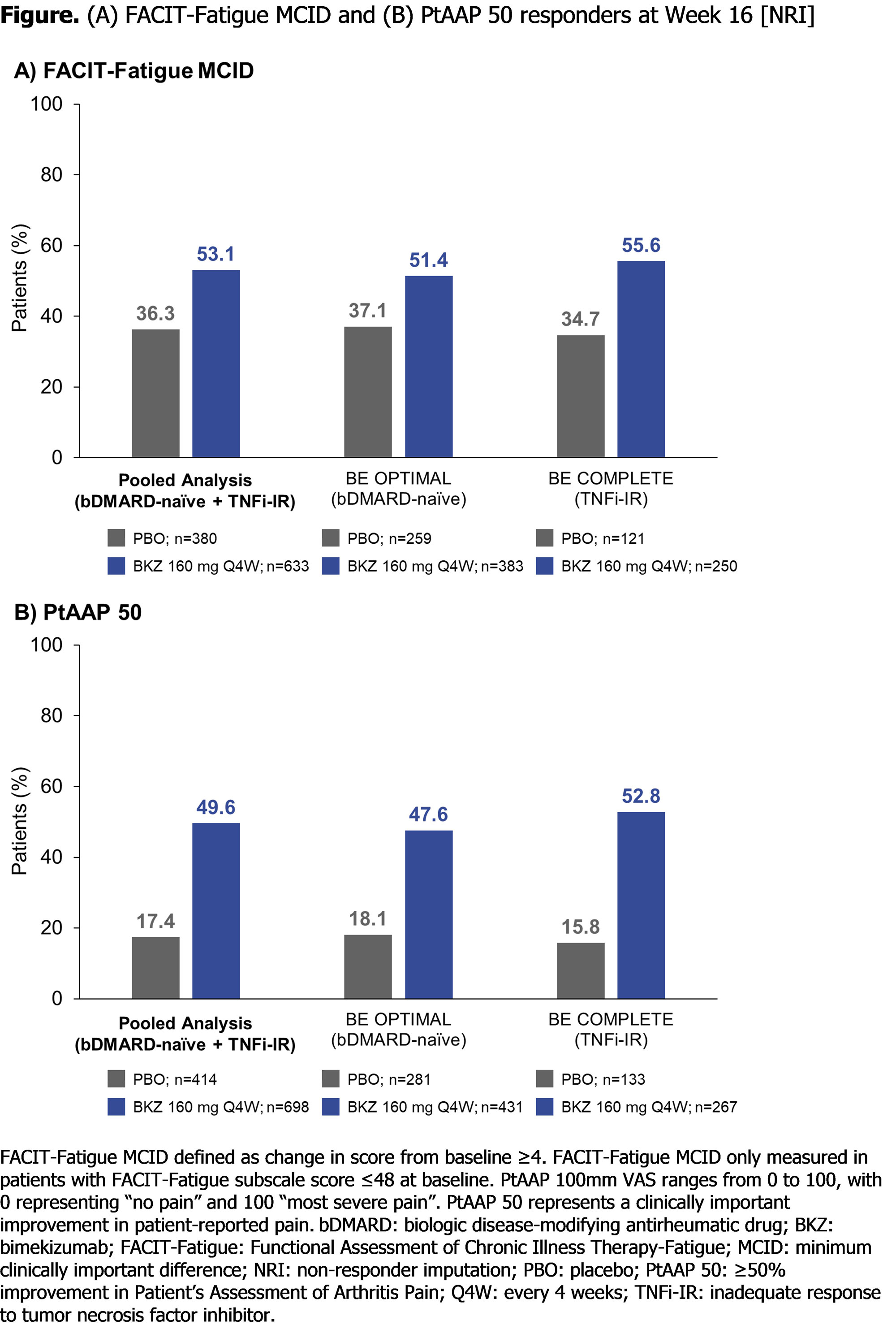
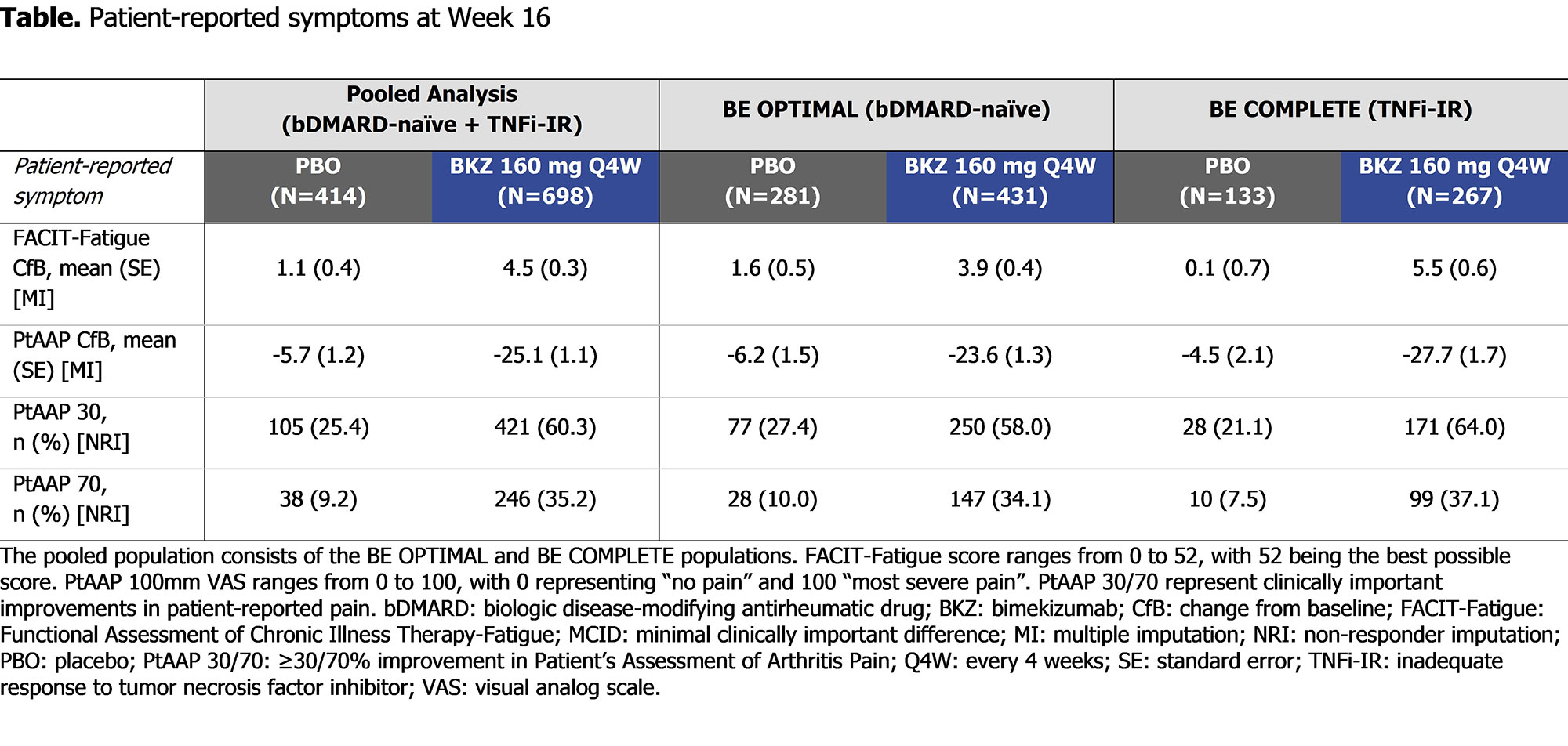
At Wk 16 (pooled), BKZ demonstrated numerically greater and clinically meaningful improvements in patient-reported fatigue and pain compared with PBO (FACIT-Fatigue MCID: 336/633 [53.1%] BKZ vs 138/380 [36.3%] PBO; PtAAP 50: 346/698 [49.6%] BKZ vs 72/414 [17.4%] PBO; Figure). BKZ-treated patients showed greater improvements in patient-reported fatigue and pain compared with PBO in the pooled population as well as the individual trial populations, meaning that results were similar between bDMARD-naïve and TNFi-IR patients (Table).
Conclusion: Bimekizumab treatment resulted in clinically meaningful improvements in patient-reported fatigue (FACIT-Fatigue) and pain (PtAAP) in approximately half of patients with active PsA who are bDMARD-naïve or TNFi-IR. Improvements were similar between bDMARD-naïve and TNFi-IR patients, suggesting that BKZ treatment leads to similar improvements in the patient-reported symptoms of fatigue and pain irrespective of prior TNFi treatment.
References: 1. Ogdie A. RMD Open 2020; 6(3):e001321; 2. Gossec L. Arthritis Rheumatol. 2021;73(suppl 10;1350); 3. Dworkin RH. J. Pain 2008; 9(2):105–21.
To cite this abstract in AMA style:
Husni M, Mease P, Merola J, Behrens F, Favalli E, McGonagle D, Tillett W, Tsuji S, Ink B, Assudani D, Bajracharya R, Coarse J, Lambert J, Gossec L. Bimekizumab Treatment Results in Improvements in Fatigue and Pain in Biologic DMARD-Naïve or TNFi-IR Patients with Active Psoriatic Arthritis: Pooled 16-Week Results from Two Phase 3 Randomized, Placebo-Controlled Studies [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/bimekizumab-treatment-results-in-improvements-in-fatigue-and-pain-in-biologic-dmard-naive-or-tnfi-ir-patients-with-active-psoriatic-arthritis-pooled-16-week-results-from-two-phase-3-randomized-place/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/bimekizumab-treatment-results-in-improvements-in-fatigue-and-pain-in-biologic-dmard-naive-or-tnfi-ir-patients-with-active-psoriatic-arthritis-pooled-16-week-results-from-two-phase-3-randomized-place/