Session Information
Date: Tuesday, October 28, 2025
Title: (2338–2376) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Bimekizumab (BKZ) is a monoclonal IgG1 antibody that selectively inhibits interleukin (IL)-17F in addition to IL-17A. The PsA Impact of Disease-12 (PsAID-12) questionnaire is a disease-specific patient (pt)-reported outcome measure that assesses impact of PsA on 12 physical, social, and psychological domains.1,2 We report long-term efficacy of BKZ treatment using the PsAID-12 questionnaire to 3 years (yrs) in pts with active PsA.
Methods: BE OPTIMAL (NCT03895203; biologic DMARD [bDMARD]-naïve) and BE COMPLETE (NCT03896581; TNF inhibitor inadequate response/intolerance [TNFi-IR]) assessed subcutaneous (sc) BKZ 160 mg every 4 weeks (wks; Q4W) in pts with PsA; both were placebo (PBO)-controlled to Wk 16. At Wk 16, PBO pts switched to BKZ (PBO/BKZ). BE OPTIMAL included a reference arm (sc adalimumab 40 mg Q2W; data not shown). BE OPTIMAL Wk 52 and BE COMPLETE Wk 16 completers could enter BE VITAL (open-label extension; NCT04009499). PsAID‑12 total and single-item domain scores range from 0–10; higher scores indicate worse status.2 Change from baseline (BL; CfB), clinically meaningful improvement response rates (≥3-point decrease from BL when respective BL PsAID-12 score ≥3) and symptom or impact severity based on PsAID-12 total score were assessed.1 PsAID-12 questionnaire was administered to Wk 148/156 (BE OPTIMAL/BE COMPLETE). Data reported for the BKZ Total group (PBO/BKZ and BKZ-randomized pts) as observed case, modified non-responder imputation (mNRI; binary) or multiple imputation (MI; continuous). mNRI considered all visits following discontinuation due to adverse events or lack of efficacy as non-response; all other missing data imputed with MI and the response derived from the imputed values.
Results: Overall, 555/712 (77.9%) bDMARD-naïve and 299/400 (74.8%) TNFi-IR BKZ Total group pts completed Wk 148/156.Achievement of clinically meaningful improvement responses in PsAID-12 total score observed at Wk 52 (49.2%) and Wk 40 (49.2%) in bDMARD-naïve and TNFi-IR pts were sustained to Wk 148 (45.6%) and Wk 156 (50.8%; Figure 1A). Clinically meaningful improvement responses were achieved by 47.5–70.7% of pts across all single-item domains at Wk 148/156. Similarly, proportions of pts achieving no/low symptom or impact severity for PsAID-12 total score (score ≤1.95) at Wk 52/40 were sustained to Wk 148/156 in 68.2% bDMARD-naïve and 68.6% TNFi-IR pts (Figure 2).Improvements from BL in PsAID-12 total score were sustained from 1 yr to 3 yrs of BKZ treatment: mean CfB (standard error) –2.3 (0.1) at Wk 52 and –2.2 (0.1) at Wk 148 in bDMARD-naïve pts; −2.4 (0.1) at Wk 40 and −2.4 (0.1) at Wk 156 in TNFi-IR pts (Figure 1B). Improvements from BL across all PsAID-12 single item domain mean scores observed at Wk 52/40 were sustained or further improved to Wk 148/156 in the BKZ Total group (Figure 3).
Conclusion: BKZ treatment resulted in clinically meaningful improvements in PsAID-12 scores across physical, social and psychological domains in pts with active PsA, indicating reduced disease impact. Improvements were sustained to 3 yrs and consistent responses were seen in pts who were bDMARD-naive or had TNFi-IR.References: 1. Gossec L. RMD Open 2024;10:e003548; 2. Gossec L. Ann Rheum Dis 2014;73:1012–9.
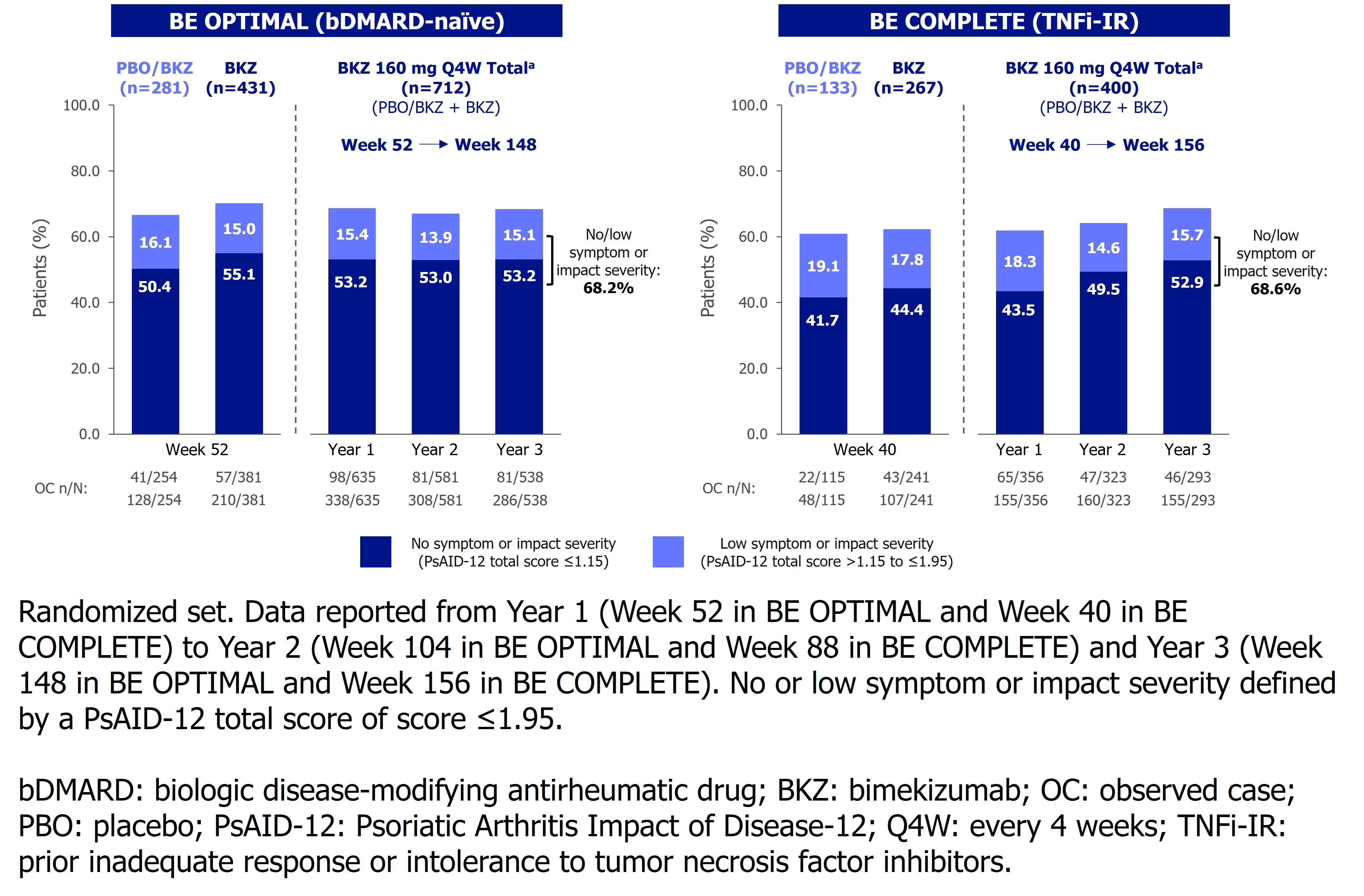 Figure 1. (A) Clinically meaningful improvement response rate and (B) PsAID-12 total score CfB to Year 3 (MI, mNRI, OC)
Figure 1. (A) Clinically meaningful improvement response rate and (B) PsAID-12 total score CfB to Year 3 (MI, mNRI, OC)
.jpg) Figure 2. No or low symptom or impact severity by visit for PsAID‑12 total score to Year 3 (OC)
Figure 2. No or low symptom or impact severity by visit for PsAID‑12 total score to Year 3 (OC)
.jpg) Figure 3. PsAID-12 single-item domain mean scores at baseline, Week 16, Year 1, and Year 3 (MI)
Figure 3. PsAID-12 single-item domain mean scores at baseline, Week 16, Year 1, and Year 3 (MI)
To cite this abstract in AMA style:
Orbai A, Gladman D, Coates L, de Wit M, Ink B, Bajracharya R, Healy P, Lambert J, Gossec L. Bimekizumab Treatment Resulted in Long-Term Sustained Reductions in Disease Impact Assessed by the Psoriatic Arthritis Impact of Disease (PsAID)-12 Questionnaire in Patients with Active Psoriatic Arthritis: Up to 3-Year Results from Two Phase 3 Studies [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/bimekizumab-treatment-resulted-in-long-term-sustained-reductions-in-disease-impact-assessed-by-the-psoriatic-arthritis-impact-of-disease-psaid-12-questionnaire-in-patients-with-active-psoriatic-arth/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/bimekizumab-treatment-resulted-in-long-term-sustained-reductions-in-disease-impact-assessed-by-the-psoriatic-arthritis-impact-of-disease-psaid-12-questionnaire-in-patients-with-active-psoriatic-arth/
