Session Information
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: The impact of bimekizumab (BKZ), a monoclonal IgG1 antibody that selectively inhibits interleukin (IL)‑17F in addition to IL-17A, on structural lesions in patients (pts) with axial spondyloarthritis (axSpA) has not yet been shown. Here, we report the impact of BKZ on MRI inflammatory and structural lesions in sacroiliac joints (SIJ) of pts with non-radiographic and radiographic (nr-/r-)axSpA to Week (Wk) 52 in the phase 3 studies BE MOBILE 1 and 2.
Methods: In BE MOBILE 1 (nr-axSpA; NCT03928704) and 2 (r-axSpA; NCT03928743), pts were randomized to BKZ 160 mg every 4 wks or placebo (PBO); all received BKZ from Wk 16–52. Spondyloarthritis Research Consortium of Canada (SPARCC) SIJ inflammation score and SPARCC SIJ Structural Scores (SSS: erosion, backfill, fat lesions, ankylosis) were assessed at baseline, Wk 16, and Wk 52 in MRI sub-studies. MRIs were assessed centrally by 2 independent experts and adjudicated for disagreements. Inflammatory and structural lesions were assessed by independent groups of central readers, hence the number of MRIs successfully scored could differ. All readers were blinded to timepoint/clinical data; structural lesions were analyzed post hoc. For pts with valid MRI assessments at all 3 timepoints, we report proportion of pts with a baseline inflammation score ≥2 achieving MRI remission (score of < 2), mean absolute inflammation scores, and change from baseline in SSS at Wks 16 and 52 (observed case).
Results: Overall, 60% (152/254) and 42% (139/332) of pts with nr- and r-axSpA were enrolled in MRI sub‑studies; 76% (115/152) and 78% (109/139) of pts had valid SPARCC SIJ inflammation assessments at all 3 timepoints, respectively, and 84% (128/152) and 83% (116/139) of pts had valid SSS assessments at all 3 timepoints.
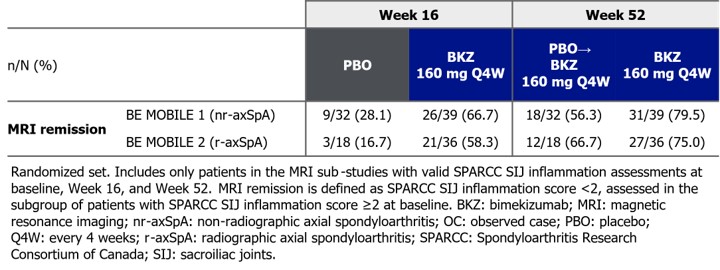
Among those with valid SPARCC SIJ inflammation assessments at all 3 timepoints and baseline inflammation scores ≥2 (nr-axSpA: PBO: 32/152 [21%], BKZ: 39/152 [26%]; r‑axSpA: PBO: 18/139 [13%], BKZ: 36/139 [26%]), a larger proportion of BKZ- vs PBO‑randomized pts achieved MRI remission at Wk 16 (Table). The proportion of continuous BKZ pts and pts switching from PBO to BKZ at Wk 16 (PBO-switchers) achieving MRI remission increased from Wk 16 to Wk 52 (Table).
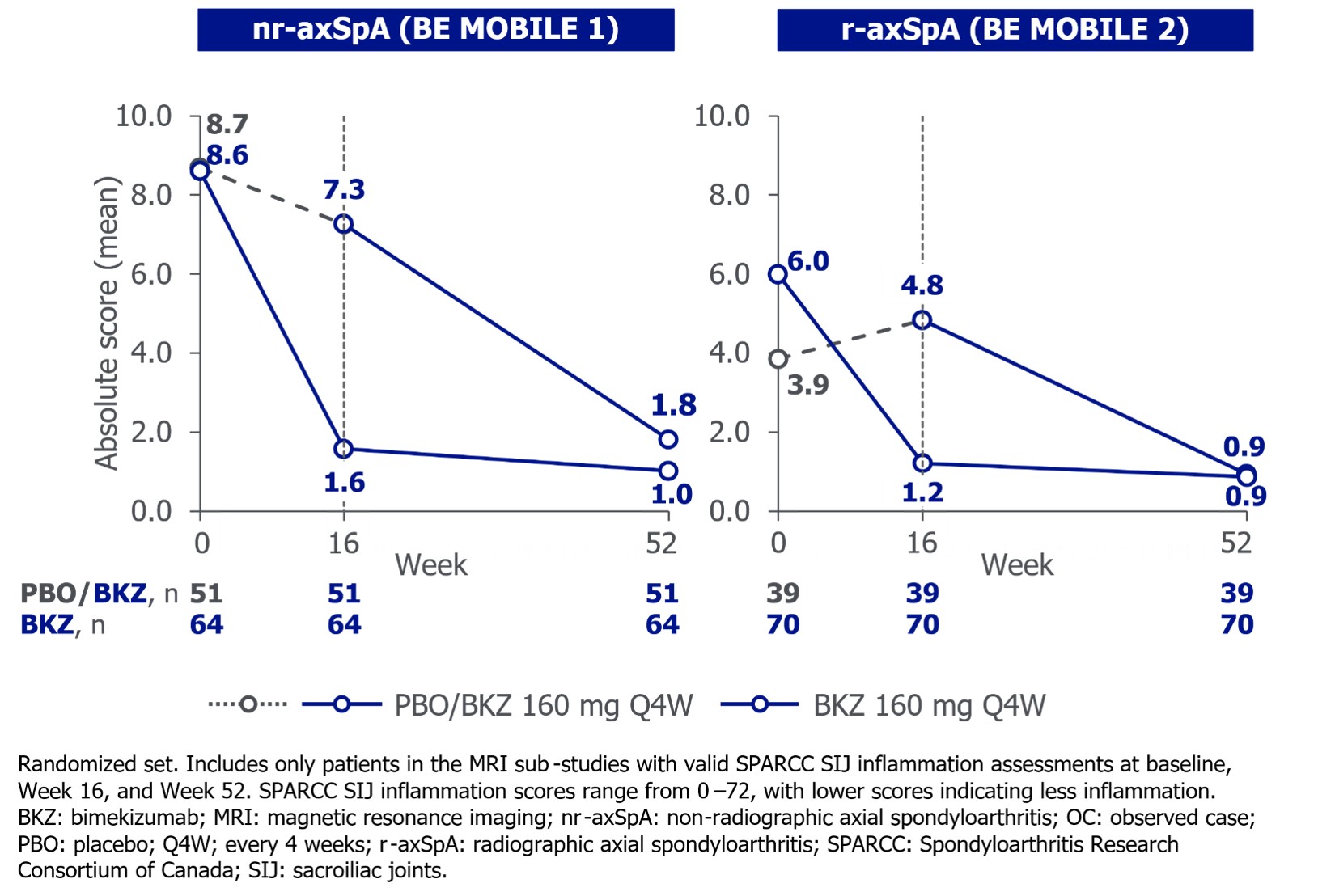
Mean baseline SPARCC SIJ inflammation scores were largely comparable between BKZ‑ and PBO‑randomized pts with nr- and r‑axSpA (Figure 1). Substantial reductions in inflammation at Wk 16 were maintained to Wk 52 for continuous BKZ pts; PBO‑switchers reached similar levels of improvement as continuous BKZ pts at Wk 52 (Figure 1).
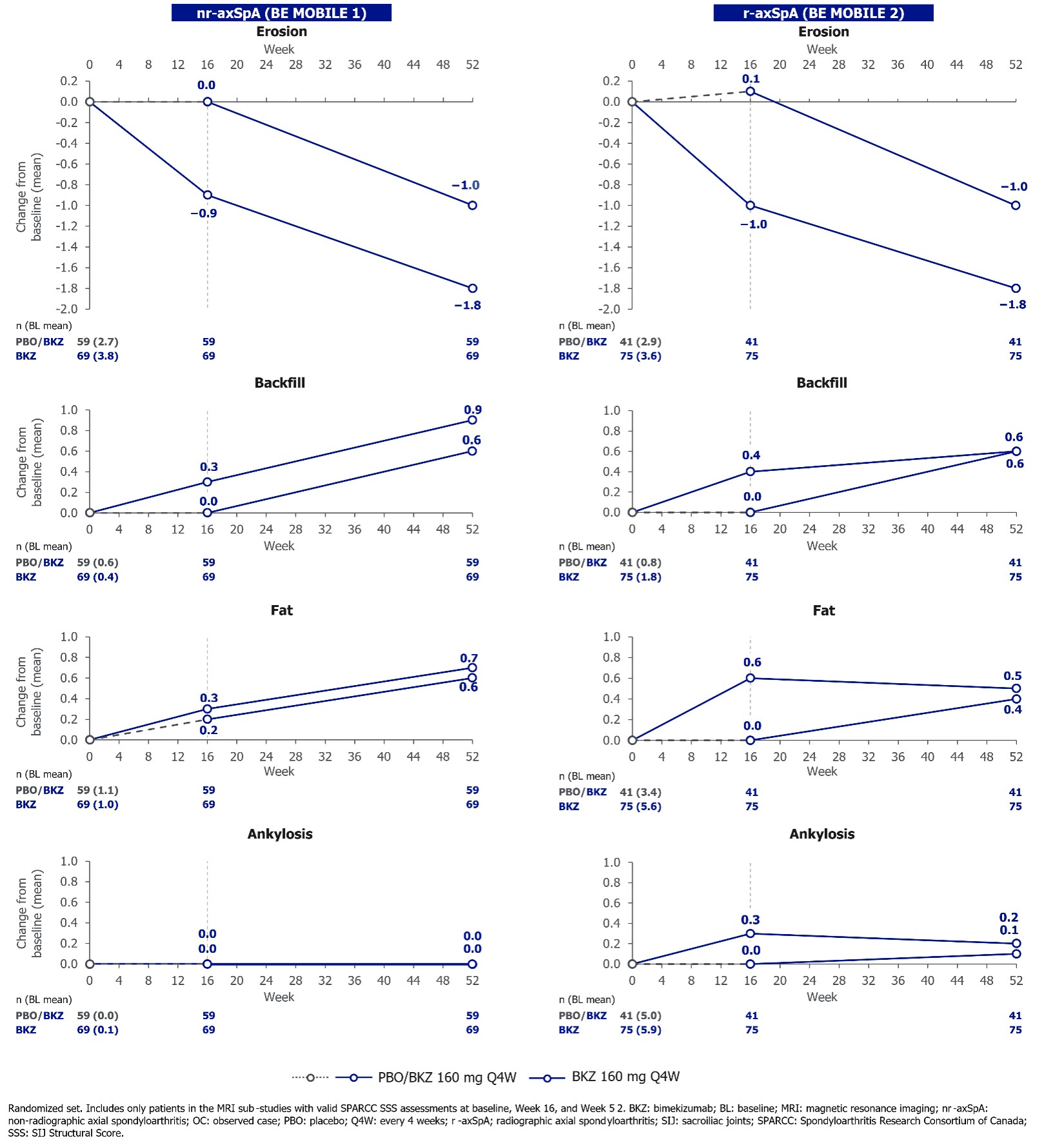
Reductions in SSS for erosions and increases in backfill and fat lesions were observed with BKZ vs PBO at Wk 16, with further improvements mostly observed in these scores to Wk 52 in continuous BKZ pts; similar changes were observed in PBO-switchers (Figure 2). No or minimal changes in SSS for ankylosis were observed following treatment with BKZ in pts with nr‑ and r-axSpA, respectively, to Wk 52 (Figure 2).
Conclusion: Across the full disease spectrum of axSpA, BKZ had substantial impact on SIJ MRI inflammation and structural lesions, indicating potential tissue repair after only 16 wks of treatment. This continued to improve from 16 to 52 wks.
To cite this abstract in AMA style:
Maksymowych W, Ramiro S, Poddubnyy D, Baraliakos X, Lambert R, Massow U, Vaux T, Prajapati C, Marten A, de Peyrecave N, Ostergaard M. Bimekizumab Treatment Resulted in Improvements in MRI Inflammatory and Structural Lesions in the Sacroiliac Joints of Patients with Axial Spondyloarthritis: 52-Week Results and Post Hoc Analyses from Two Phase 3 Studies [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/bimekizumab-treatment-resulted-in-improvements-in-mri-inflammatory-and-structural-lesions-in-the-sacroiliac-joints-of-patients-with-axial-spondyloarthritis-52-week-results-and-post-hoc-analyses-from/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/bimekizumab-treatment-resulted-in-improvements-in-mri-inflammatory-and-structural-lesions-in-the-sacroiliac-joints-of-patients-with-axial-spondyloarthritis-52-week-results-and-post-hoc-analyses-from/