Session Information
Date: Tuesday, November 14, 2023
Title: (2227–2256) Spondyloarthritis Including Psoriatic Arthritis – Treatment: SpA Poster III
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Bimekizumab (BKZ), a monoclonal IgG1 antibody that selectively inhibits IL-17F in addition to IL-17A, has shown superior efficacy to 16 weeks (wks) vs placebo (PBO) and tolerability in patients (pts) with active PsA in the phase 3 BE OPTIMAL and BE COMPLETE studies.1,2 Efficacy of BKZ to 52 wks has also been demonstrated in the BE OPTIMAL study in biologic-naïve pts with PsA.3 Here, the efficacy and safety of BKZ treatment in pts with active PsA and prior inadequate response or intolerance to TNF inhibitors (TNFi-IR) are reported up to Wk 52 in the BE COMPLETE study.
Methods: BE COMPLETE (NCT03896581) included a 16-wk double-blind, PBO-controlled period. Wk 16 completers were eligible for entry into BE VITAL (NCT04009499; open-label extension). Pts were randomized 2:1 to subcutaneous BKZ 160 mg every 4 wks or PBO. At Wk 16, PBO pts switched to BKZ (PBO/BKZ; received 36 wks of BKZ treatment up to Wk 52). BE VITAL included pts from BE OPTIMAL and BE COMPLETE; data here are for pts randomized at baseline (BL [Wk 0]) of BE COMPLETE only, up to 52 wks. Efficacy data are reported as observed case (OC) or using non‑responder imputation (NRI; binary) or multiple imputation (MI; continuous). The number of treatment-emergent adverse events (TEAEs) to Wk 52 are reported for pts who received ≥1 dose of BKZ.
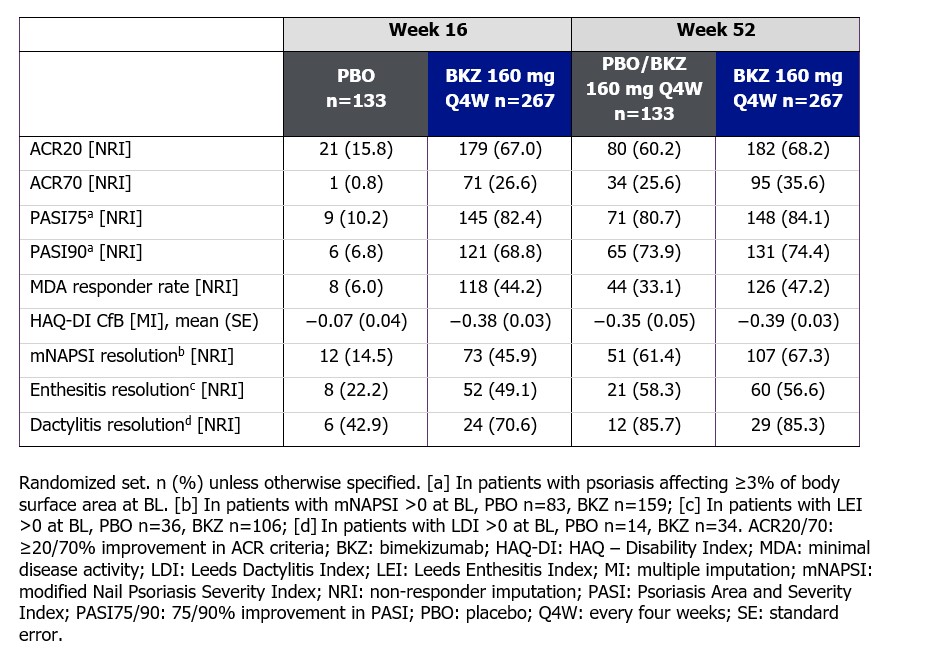
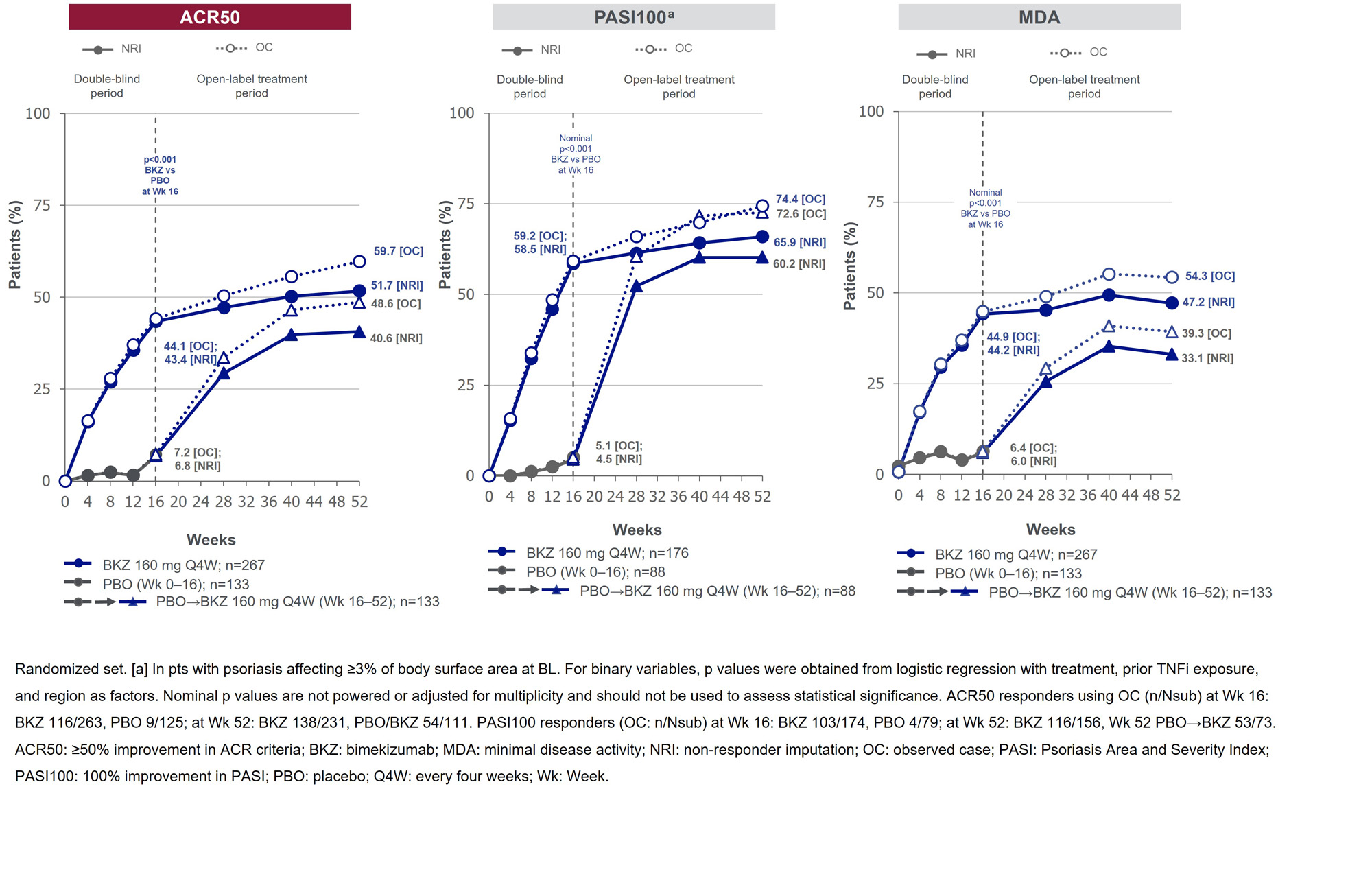
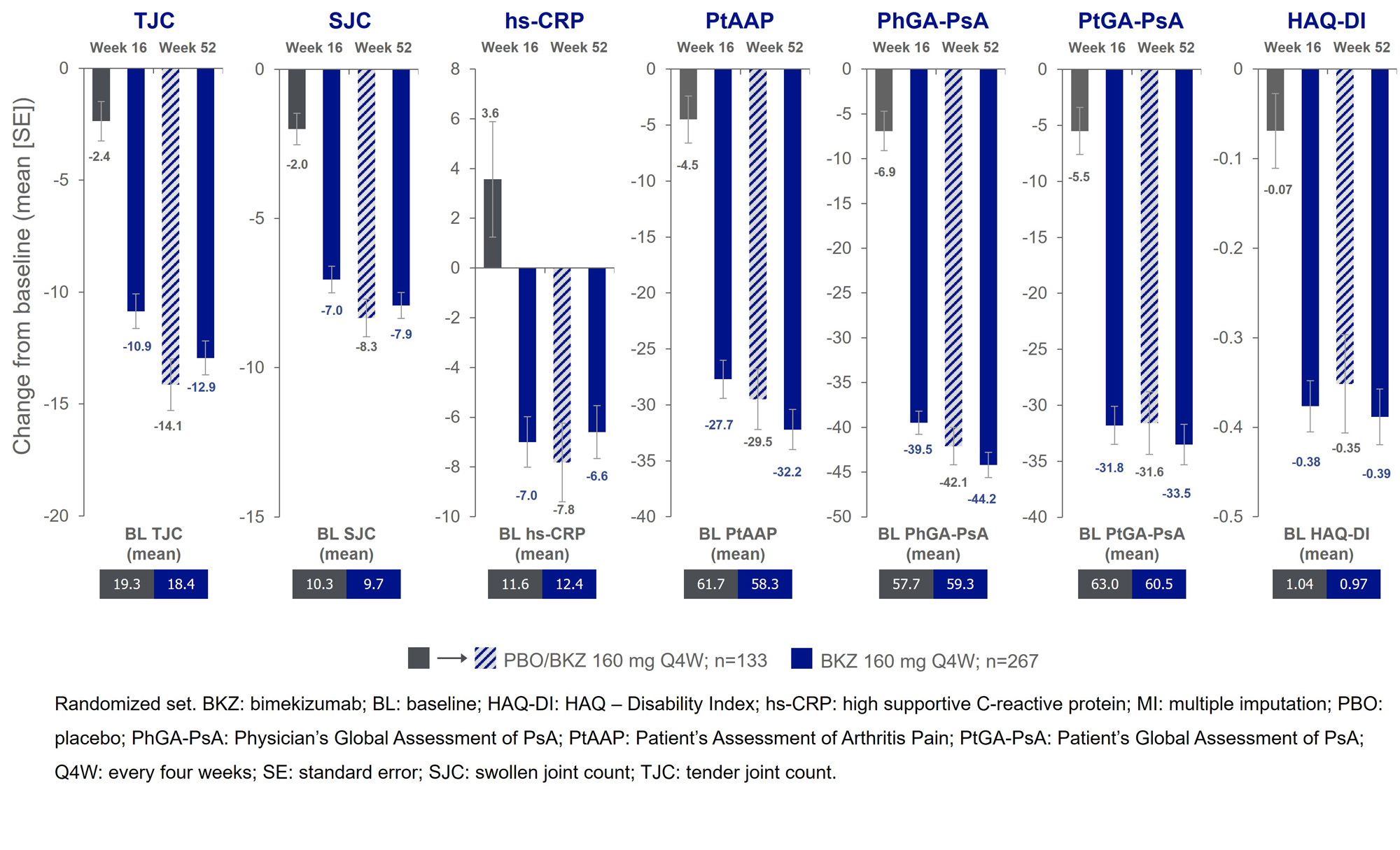
Results: 388/400 (97.0%) pts completed Wk 16; 377 (94.3%) entered BE VITAL and 347 (86.8%) completed Wk 52. Improved joint and skin efficacy responses with BKZ treatment were sustained from Wk 16 to Wk 52 (Table). At Wk 52, 138/267 (51.7%) of BKZ and 54/133 (40.6%) of PBO/BKZ pts achieved ACR50 (Figure 1). Improvements from BL in all ACR components were seen at Wk 16 and sustained to Wk 52 in BKZ-treated pts (Figure 2). In pts with BL psoriasis (≥3% body surface area), 116/176 (65.9%) of BKZ and 53/88 (60.2%) of PBO/BKZ pts achieved complete skin clearance (Psoriasis Area Severity Index [PASI]100) at Wk 52. At Wk 52, 126/267 (47.2%) of BKZ and 44/133 (33.1%) of PBO/BKZ pts achieved minimal disease activity (MDA; Figure 1). To Wk 52, 243/388 (62.6%) pts had ≥1 TEAE whilst receiving BKZ (exposure-adjusted incidence rate per 100 pt-years [EAIR/100 PY]: 126.0); 23 (5.9%) pts reported a serious TEAE (7.0/100 PY). Malignancies (excluding nonmelanoma skin cancers) were reported by 2 (0.7%) pts receiving BKZ (0.77/100 PY). Candida infections were reported by 25 (6.4%) pts receiving BKZ (7.7/100 PY); all were reported as mild or moderate by investigators and none were systemic. Two cases of oral candidiasis led to study discontinuation. There was one death (sudden death; pt with history of cardiac events), two adjudicated major adverse cardiac events and no definite or probable adjudicated inflammatory bowel disease.
Conclusion: In pts with PsA and TNFi-IR, BKZ demonstrated sustained clinical efficacy from Wk 16 up to Wk 52. The safety profile was consistent with previous reports.1–3
References: 1. McInnes IB. Lancet 2023; 401(25–37); 2. Merola JF. Lancet 2023; 401(38–48); 3. Ritchlin C. Arthritis Rheumatol 2022;74(S9).
To cite this abstract in AMA style:
Coates L, Landewé R, McInnes I, Mease P, Ritchlin C, Tanaka Y, Asahina A, Behrens F, Gladman D, Gossec L, Gottlieb A, Warren R, Ink B, Bajracharya R, Coarse J, Merola J. Bimekizumab Treatment in Patients with Active PsA and Prior Inadequate Response to TNF Inhibitors: Sustained Efficacy and Safety Results from a Phase 3 Study and Its Open-Label Extension up to 1 Year [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/bimekizumab-treatment-in-patients-with-active-psa-and-prior-inadequate-response-to-tnf-inhibitors-sustained-efficacy-and-safety-results-from-a-phase-3-study-and-its-open-label-extension-up-to-1-year/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/bimekizumab-treatment-in-patients-with-active-psa-and-prior-inadequate-response-to-tnf-inhibitors-sustained-efficacy-and-safety-results-from-a-phase-3-study-and-its-open-label-extension-up-to-1-year/