Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: PsA significantly impacts patients’ quality of life due to functional limitations, pain, and fatigue.1 Sustained relief from pain and fatigue are therefore important aims of treatment for patients with PsA. Bimekizumab (BKZ), a monoclonal IgG1 antibody that selectively inhibits interleukin (IL)-17F in addition to IL-17A, has demonstrated sustained improvements in pain and fatigue to 1 year in patients with active PsA.2 We report assessments of patient‑reported pain and fatigue up to 2 years in patients with PsA treated with BKZ.
Methods: The BE OPTIMAL (NCT03895203) and BE COMPLETE (NCT03896581) phase 3 studies assessed subcutaneous BKZ 160 mg every 4 weeks (wks) in patients with PsA who were biologic DMARD (bDMARD)-naïve or had inadequate response or intolerance to TNF inhibitors (TNFi-IR). Both had a 16-wk double-blind, placebo (PBO)‑controlled period; BE OPTIMAL included a reference arm (adalimumab [ADA] 40 mg every 2 wks). Patients who completed Wk 52 of BE OPTIMAL and Wk 16 of BE COMPLETE were eligible for the open-label extension, BE VITAL (NCT04009499), in which all patients received BKZ. There was no washout period for patients who switched from ADA to BKZ (ADA/BKZ).
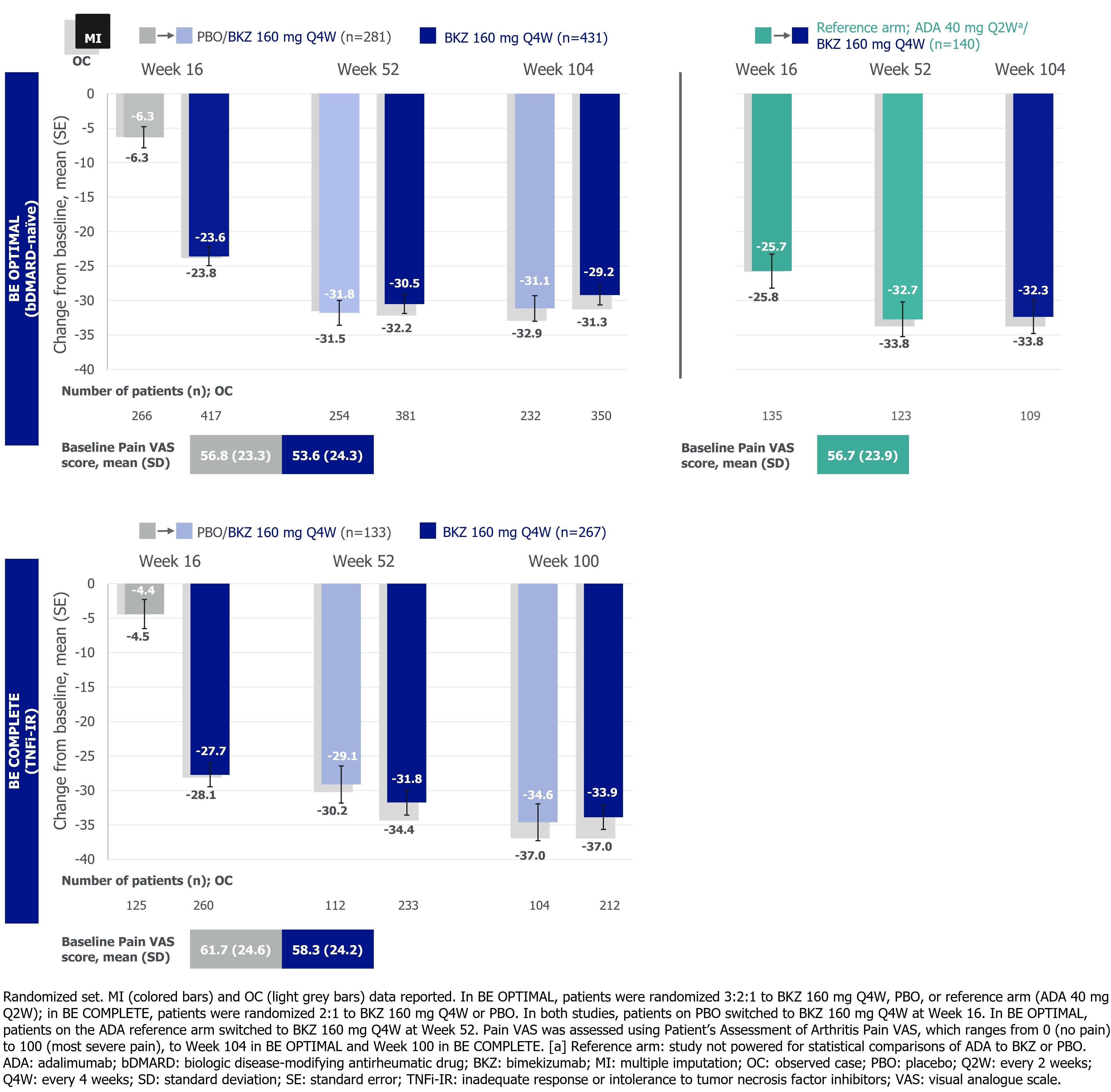
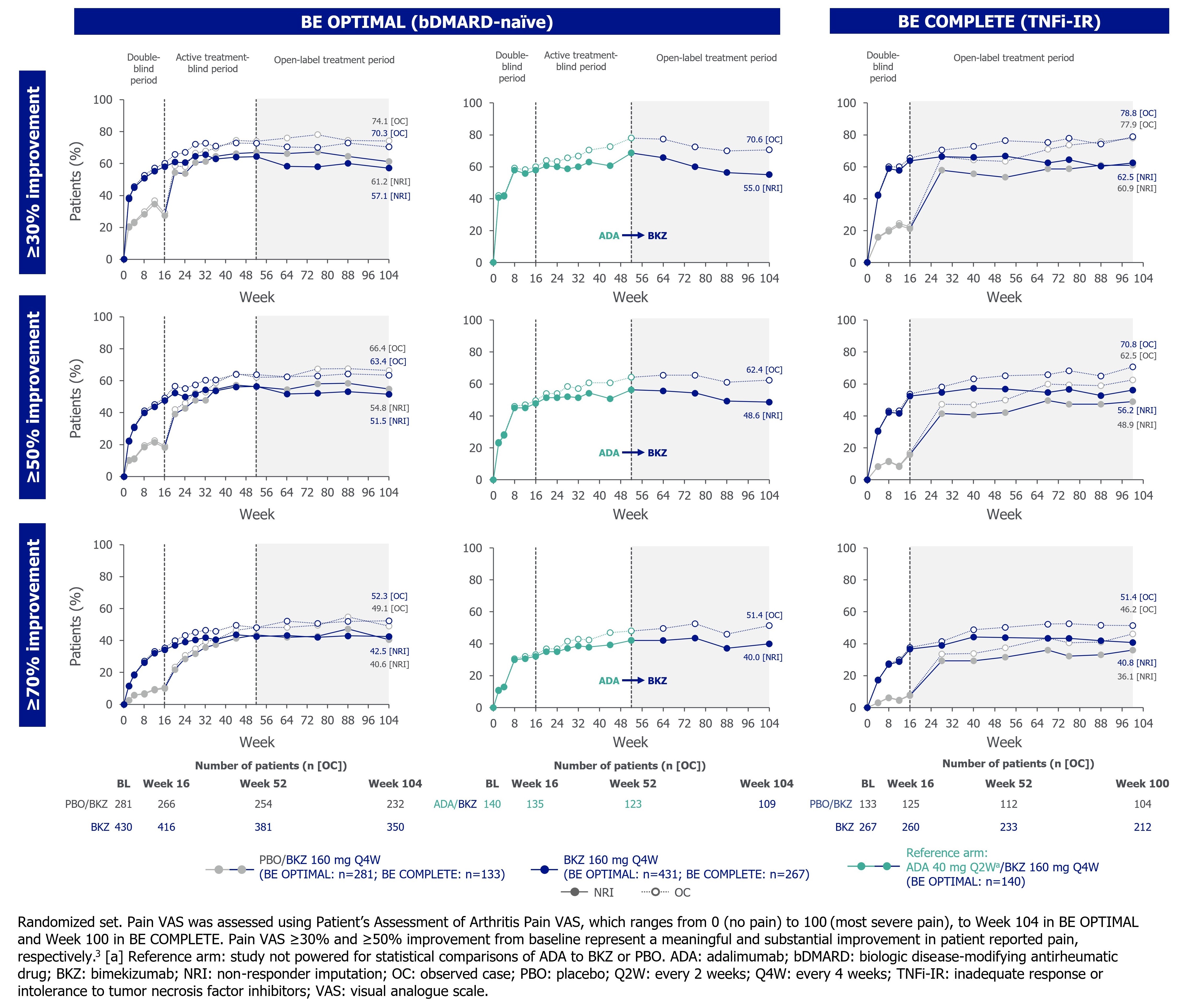
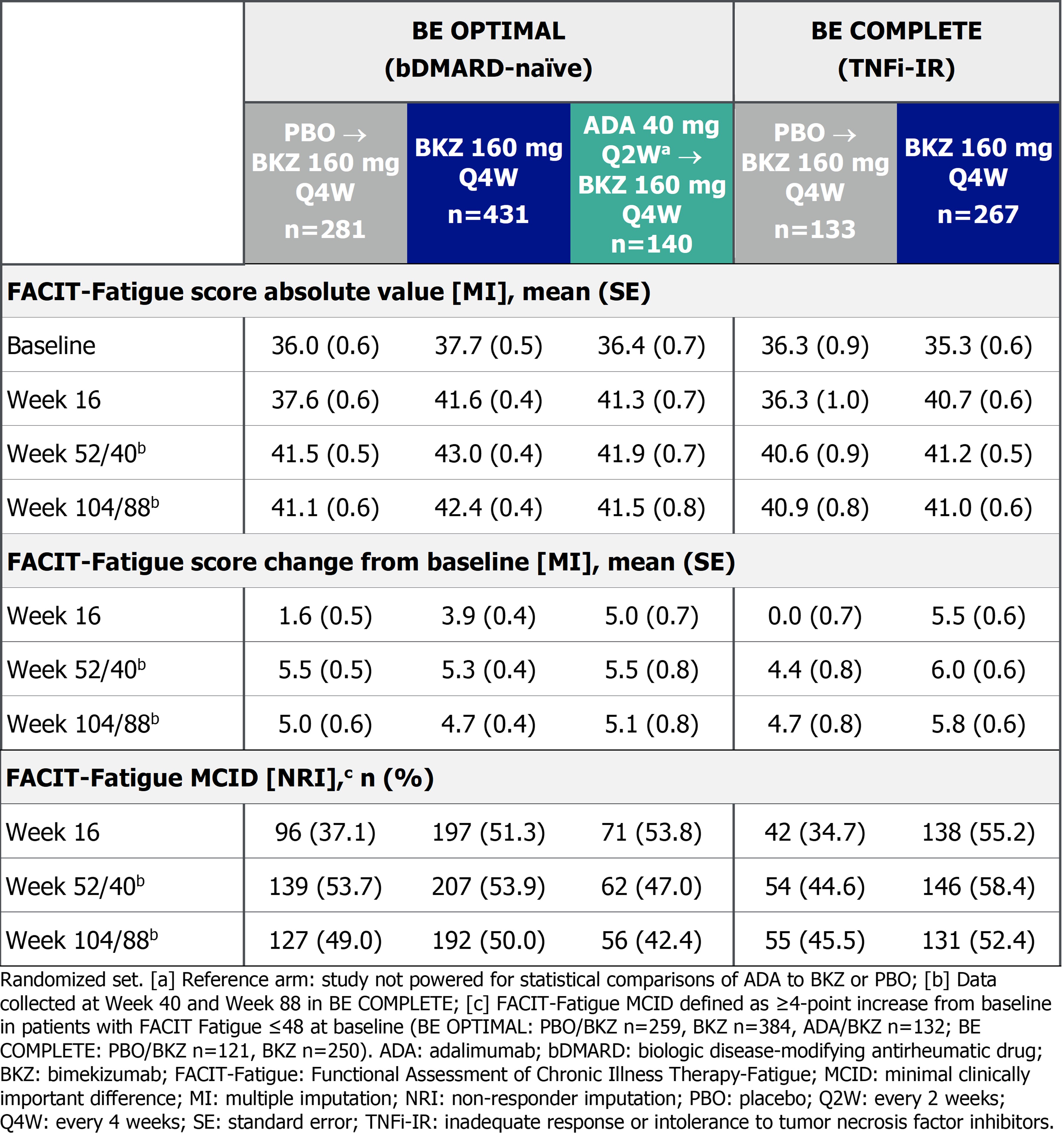
Arthritis pain was assessed using Patient’s Assessment of Arthritis Pain Visual Analogue Scale (Pain VAS; 0 [no pain] to 100 [most severe pain]) to Wk 104 in BE OPTIMAL and Wk 100 in BE COMPLETE. Fatigue was assessed using the Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-Fatigue) subscale (0 [worst] to 52 [best]) to Wk 104 in BE OPTIMAL and Wk 88 in BE COMPLETE. Pain VAS change from baseline (BL; CfB) and clinically important improvements from baseline (≥30/50/70%)3 and FACIT‑Fatigue CfB and minimal clinically important difference responder rates (MCID; ≥4-point improvement from BL in patients with FACIT‑Fatigue ≤48 at BL) are reported here. Data reported as observed and using non‑responder imputation (binary) or multiple imputation (continuous).
Results: 710/852 (83.3%) and 322/400 (80.5%) patients completed Wk 104/100 of BE OPTIMAL and BE COMPLETE. Improvements in pain achieved at 1 year were sustained up to 2 years in PBO/BKZ and BKZ-randomized patients (Figure 1–2). Approximately half of patients in all treatment groups achieved a ≥50% reduction in Pain VAS at Wk 104/100. Similarly, improvements in fatigue outcomes achieved at 1 year were sustained up to 2 years in PBO/BKZ and BKZ-randomized patients (Table). For patients who switched from ADA to BKZ at Wk 52, pain and fatigue responses were sustained up to 2 years (Figure 1–2; Table).
Conclusion: Treatment with BKZ demonstrated sustained improvements to 2 years in patient‑reported pain and fatigue, symptoms that are associated with active PsA. Similar improvements were observed irrespective of prior bDMARD treatment. Patients who switched from ADA to BKZ at Wk 52 showed sustained improvements in pain and fatigue up to 2 years.
References: 1. Ogdie A. RMD Open 2020;6:e001321; 2. Husni ME. ACR 2023 (Poster 0527); 3. Dworkin RH. J Pain 2008;9:105–21.
To cite this abstract in AMA style:
Mease P, Tillett W, de Wit M, Gossec L, Husni M, Proft F, Ink B, Bajracharya R, Coarse J, Lambert J, Coates L, Gottlieb A. Bimekizumab-Treated Patients with Active Psoriatic Arthritis Showed Sustained Improvements in Pain and Fatigue: Up to 2-Year Results from Two Phase 3 Studies [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/bimekizumab-treated-patients-with-active-psoriatic-arthritis-showed-sustained-improvements-in-pain-and-fatigue-up-to-2-year-results-from-two-phase-3-studies/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/bimekizumab-treated-patients-with-active-psoriatic-arthritis-showed-sustained-improvements-in-pain-and-fatigue-up-to-2-year-results-from-two-phase-3-studies/