Session Information
Date: Sunday, November 7, 2021
Title: Spondyloarthritis Including PsA – Treatment Poster I: Axial Spondyloarthritis (0908–0939)
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: Bimekizumab (BKZ), a monoclonal IgG1 antibody that selectively inhibits both interleukin (IL)-17F and IL-17A, has been demonstrated to be efficacious and well tolerated in patients (pts) with ankylosing spondylitis (AS) treated for up to 3 years (yrs).1–3 We report 3-yr interim health-related quality of life (HRQoL) in pts with active AS from a 1-yr phase 2b study (BE AGILE; NCT02963506) and its ongoing 4-yr open-label extension (OLE; NCT03355573).
Methods: The BE AGILE study design has been described previously.1 Following the 12‑week (wk) double-blind, placebo-controlled dose-ranging period, pts received BKZ 160 mg or 320 mg every 4 wks (Q4W) in the dose-blind period to Wk 48. Pts completing Wk 48 were eligible to enter the OLE where all pts received BKZ 160 mg Q4W. We report HRQoL following a total of 3 yrs of treatment for the BE AGILE full analysis set (all randomized patients who received ≥1 dose of investigational medicinal product and had a valid measurement of the ASAS components at baseline). Change over time in ASQoL and SF-36 scores are reported using multiple imputation (MI) and observed case (OC) methodology. Additionally, for ASQoL, we report the proportion of pts achieving a pt acceptable symptom state (PASS) of < 6.5 and a reduction from baseline greater than a minimal clinically important difference (MCID) of 3;4,5 for SF-36 PCS and MCS scores, we report the proportion of pts with an improvement in score greater than an MCID of 5.6 These analyses utilized non-responder imputation (NRI) and OC methodology; for NRI, pts who did not enter the OLE were considered non-responders from Wk 48 onwards.
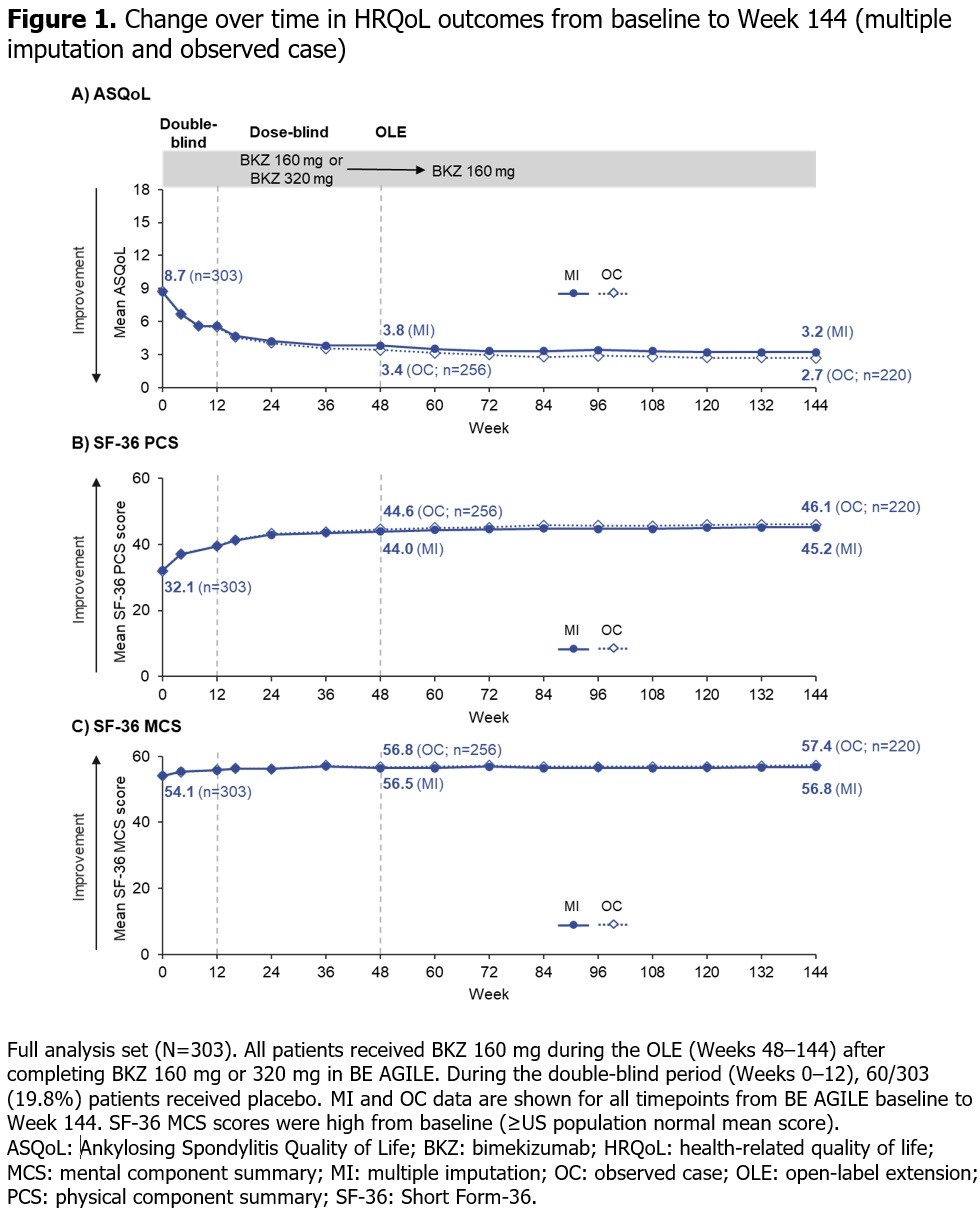
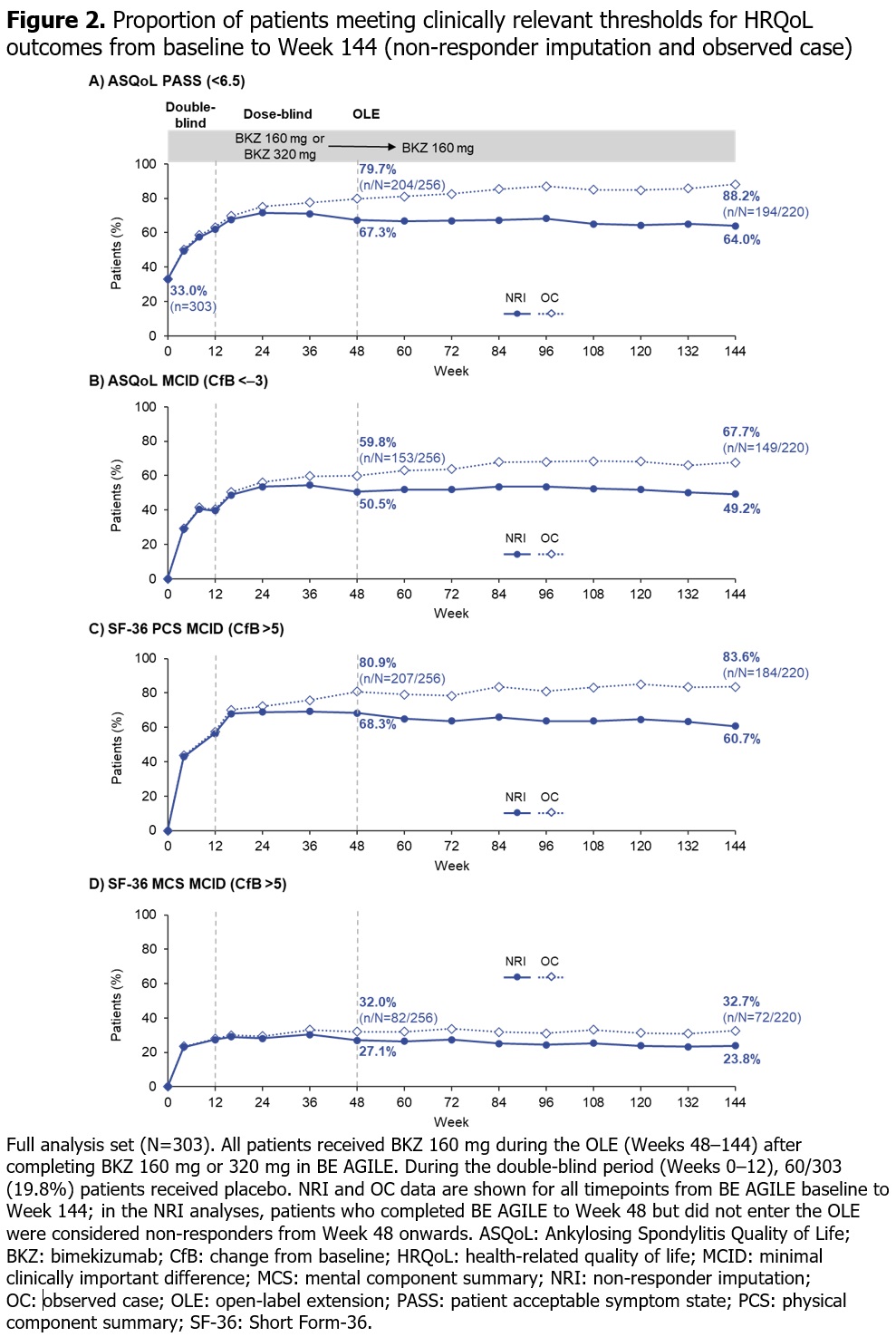
Results: 296/303 (97.7%) pts randomized at BE AGILE study baseline entered the dose-blind period at Wk 12; 262/303 (86.5%) pts completed Wk 48 on BKZ. At Wk 48, 256/303 (84.5%) pts entered the OLE, of whom 255 received BKZ 160 mg Q4W; 220/303 (72.6%) pts completed the HRQoL instruments at Wk 144. ASQoL and SF-36 PCS scores at baseline were indicative of impaired physical function in this patient population with long-standing disease; mean scores (both OC and MI) improved up to Wk 48 and these improvements were maintained up to Wk 144 (Figure 1A, B). Baseline SF-36 MCS mean was indicative of non-impaired psychological function; the slight improvement trend shown at Wk 48 was maintained over 144 wks of BKZ treatment (Figure 1C). At baseline, 33.0% pts were in a PASS for ASQoL (score < 6.5); this increased to 67.3% and 64.0% at Wk 48 and Wk 144 respectively in the NRI analysis, and 79.7% and 88.2% in the OC analysis (Figure 2A). The proportion of pts with clinically relevant improvements (≥MCID) was maintained from Wk 48 to Wk 144 for ASQoL (NRI: 50.5% to 49.2%; OC: 59.8% to 67.7%), as well as SF-36 PCS (NRI: 68.3% to 60.7%; OC: 80.9% to 83.6%) and MCS (NRI: 27.1% to 23.8%; OC: 32.0% to 32.7%; Figure 2B–D).
Conclusion: Clinically relevant improvements in HRQoL demonstrated at Wk 48 were sustained over 144 wks of BKZ treatment.
References: 1. van der Heijde D. Ann Rheum Dis 2020;79:595–604;
2. Baraliakos X. Arthritis Rheumatol 2020;72 (suppl 10):1364;
3. van der Heijde D. Ann Rheum Dis 2021;80 (suppl 1):332–3;
4. Maksymowych W.P. Arthritis Rheum 2007;57(1):133–9;
5. Richard N. Ann Rheum Dis 2018;77:645;
6. Reveille J.D. Value Health 2020;23(10):1281–5.
To cite this abstract in AMA style:
Deodhar A, Dougados M, Gaffney K, Sengupta R, Magrey M, de Peyrecave N, Oortgiesen M, Vaux T, Fleurinck C, Taieb V, de la Loge C, Baraliakos X. Bimekizumab Shows Sustained and Meaningful Long-Term Improvements in Health-Related Quality of Life in Ankylosing Spondylitis: Interim Results After 3 Years of Treatment in an Ongoing Phase 2b Study [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/bimekizumab-shows-sustained-and-meaningful-long-term-improvements-in-health-related-quality-of-life-in-ankylosing-spondylitis-interim-results-after-3-years-of-treatment-in-an-ongoing-phase-2b-study/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/bimekizumab-shows-sustained-and-meaningful-long-term-improvements-in-health-related-quality-of-life-in-ankylosing-spondylitis-interim-results-after-3-years-of-treatment-in-an-ongoing-phase-2b-study/