Session Information
Date: Sunday, November 8, 2020
Title: Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster III
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Bimekizumab (BKZ), a monoclonal antibody that selectively inhibits interleukin (IL)-17A and IL-17F, has demonstrated clinical efficacy in patients with ankylosing spondylitis (AS) treated over a period of 48 weeks.1 We report 2-year interim efficacy and safety of BKZ in patients with active AS from a phase 2b dose-ranging study (BE AGILE; NCT02963506) and ongoing open-label extension (OLE; NCT03355573).
Methods: BE AGILE consisted of a 12-week dose-ranging period followed by a 36-week randomized period (BKZ 160 or 320 mg); full study design is described elsewhere.1 Patients who completed 48 weeks’ treatment in BE AGILE were eligible for entry into the OLE. All OLE patients received BKZ 160 mg every 4 weeks (Q4W) irrespective of previous dosing regimen. Efficacy and safety outcomes are presented from BE AGILE study baseline to Week 96. Efficacy outcomes are reported for the OLE Full Analysis Set (patients who had ≥1 dose of BKZ and ≥1 valid efficacy variable measurement since entry into the OLE), and include: Assessment of SpondyloArthritis international Society (ASAS) 40% and 20% response (ASAS40/20), ASAS partial remission (ASAS PR), Ankylosing Spondylitis Disease Activity Score (ASDAS) < 2.1, ASDAS inactive disease (ID: ASDAS < 1.3), ASDAS-CRP, Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), Bath Ankylosing Spondylitis Metrology Index (BASMI) and Bath Ankylosing Spondylitis Functional Index (BASFI). Missing data were imputed using non-responder imputation (binary variables) or multiple imputation (continuous variables). Treatment-emergent adverse events (TEAEs) up to the 96-week treatment period cut-off and beyond are reported separately for the BE AGILE Safety Set and OLE Safety Set (patients who received ≥1 dose of BKZ on study entry).
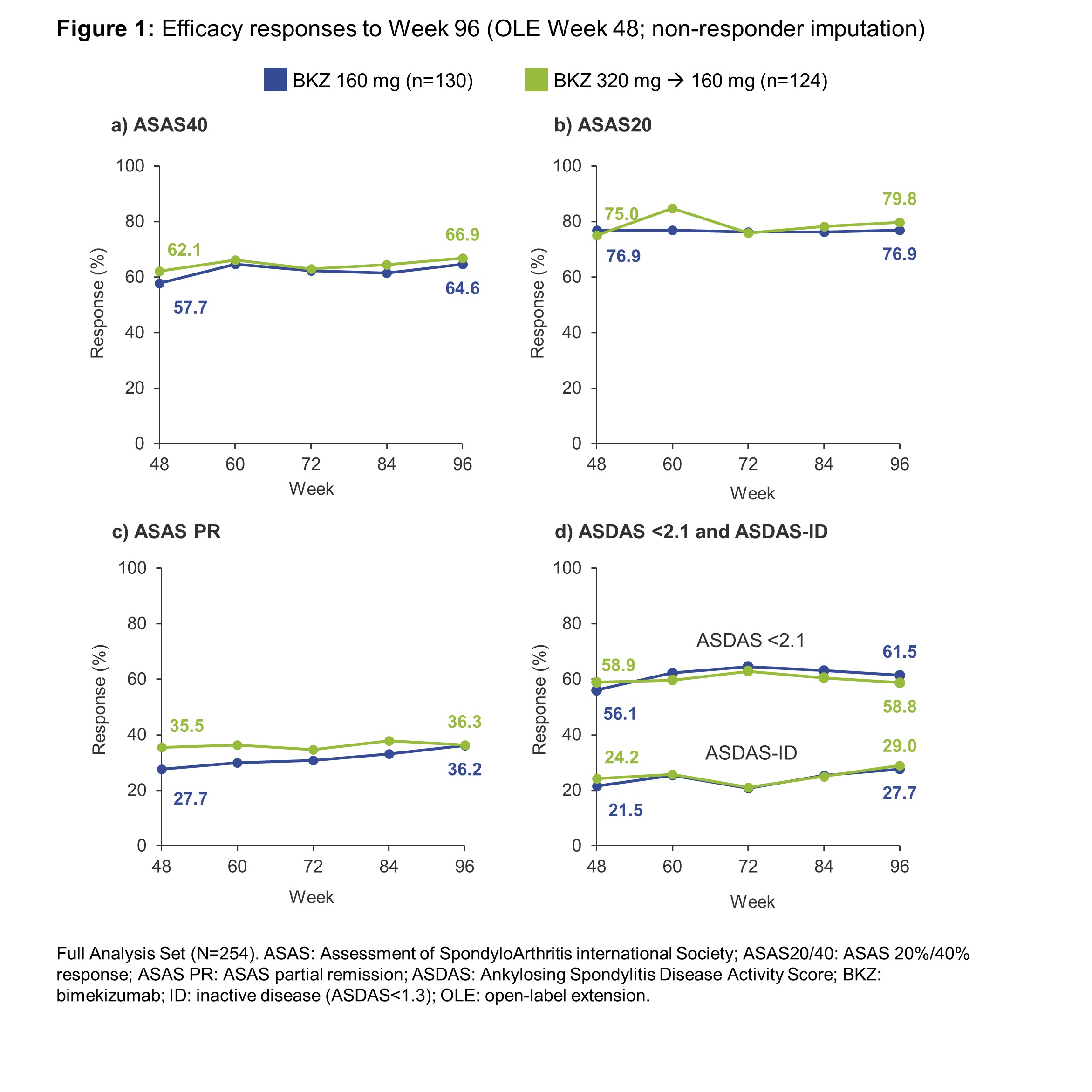
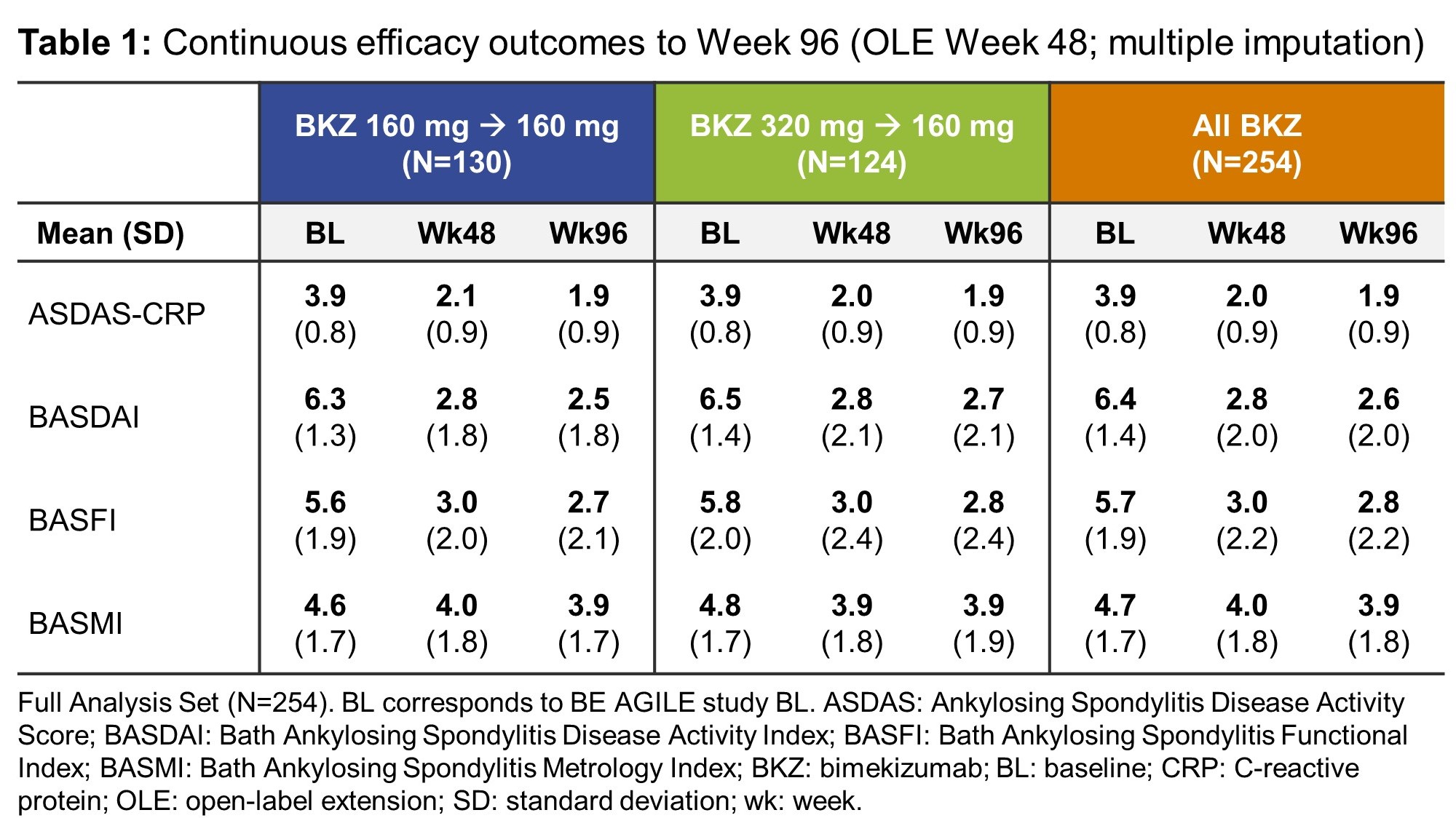
Results: 262/303 (87%) patients randomized at BE AGILE study baseline completed Week 48, of whom 254/262 (97%) were included in the OLE Full Analysis Set, including 130 who continued on BKZ 160 mg Q4W and 124 dose-reduced from BKZ 320 mg Q4W to BKZ 160 mg Q4W. Of these, 238 (94%) had an efficacy assessment at Week 96. In BE AGILE, rapid improvements in efficacy outcomes were observed in BKZ-treated patients at Week 12; these further increased to Week 48 and were maintained during the OLE from Week 48–96 (Figure 1, Table 1). Responses were similar for patients on BKZ 160 mg and 320 mg at Week 48; and remained similar between patients continuing on BKZ 160 mg and those dose-reduced from BKZ 320 mg to 160 mg up to Week 96 (Figure 1). The exposure-adjusted incidence rate (EAIR) per 100 patient-years (PY) of TEAEs was 186.2 in BE AGILE (Weeks 0–48) and 111.7 in the OLE (Week 48 onwards); for serious TEAEs the EAIR/100 PY was 5.1 and 6.1, respectively (Table 2).
Conclusion: In patients with active AS who completed the first 48 weeks of BKZ treatment in BE AGILE, BKZ provides further sustained long-term improvements in key efficacy outcome measures over 96 weeks of treatment. Dose reduction from 320 mg to 160 mg Q4W was not followed by loss of response. There were no unexpected safety findings versus previous studies.
Reference: 1. van der Heijde D. Ann Rheum Dis 2020;79:595–604.
To cite this abstract in AMA style:
Baraliakos X, Deodhar A, Dougados M, Oortgiesen M, de Peyrecave N, Bauer M, Vaux T, Fleurinck C, van der Heijde D. Bimekizumab Long-Term Efficacy and Safety over 96 Weeks in Patients with Ankylosing Spondylitis: Interim Results from a Phase 2b Open-Label Extension Study [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/bimekizumab-long-term-efficacy-and-safety-over-96-weeks-in-patients-with-ankylosing-spondylitis-interim-results-from-a-phase-2b-open-label-extension-study/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/bimekizumab-long-term-efficacy-and-safety-over-96-weeks-in-patients-with-ankylosing-spondylitis-interim-results-from-a-phase-2b-open-label-extension-study/