Session Information
Date: Monday, November 8, 2021
Title: Spondyloarthritis Including PsA – Treatment Poster II: Psoriatic Arthritis I (1329–1363)
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
Background/Purpose: Bimekizumab (BKZ) is a monoclonal IgG1 antibody that selectively inhibits interleukin (IL)-17F and IL-17A, and has demonstrated clinical improvements in joint and skin outcomes up to 152 weeks (wks) and an acceptable safety profile in patients (pts) with active psoriatic arthritis (PsA).1,2 We report the long-term efficacy and safety of BKZ treatment up to 3 years in the overall BKZ-treated population and TNFi-naïve (n) pts from a phase 2b dose-ranging study (BE ACTIVE; NCT02969525) and its open-label extension (OLE; NCT03347110).
Methods: BE ACTIVE and OLE study designs have been described previously.1,2 Pts who completed 48 wks of BKZ treatment without meeting withdrawal criteria were eligible for OLE entry. All OLE pts received BKZ 160 mg every 4 wks after completing BKZ 160 mg or 320 mg in BE ACTIVE. Data are presented from Wk 48 to 152 for all BKZ-treated pts and the TNFi-n (defined as biologic DMARD-naïve) subgroup. Pre-planned subgroup analyses of efficacy outcomes are reported for the full analysis set (FAS; pts who received ≥1 dose BKZ with a valid primary efficacy variable measurement at baseline [BL]): ACR criteria 20/50/70; resolution of dactylitis (in pts with BL Leeds Dactylitis Index >0) or enthesitis (in pts with BL Maastricht Ankylosing Spondylitis Enthesitis Score >0); Psoriasis Area and Severity Index (PASI)100 (in pts with BL body surface area ≥3%); dual achievement of ACR50+PASI100; and minimal/very low disease activity (MDA/VLDA) in PsA. Treatment-emergent adverse events (TEAEs) are reported for the safety set (SS; pts who received ≥1 dose BKZ in BE ACTIVE).
Results: In BE ACTIVE, 206 pts were randomized at BL and 184 were enrolled in the OLE.1,2 Of pts randomized at BL, 167 were TNFi-n. Over half of TNFi-n pts achieved ACR50 at Wks 48/152 (58.7/52.7% non-responder imputation [NRI]; 63.6/69.8% observed case [OC]; Table 1; Figure 1). At Wk 152, the majority of TNFi-n pts had resolution of dactylitis (70.2% NRI; 100.0% OC) and enthesitis (60.7% NRI; 80.6% OC). The proportion of TNFi-n pts with PASI100 at Wks 48/152 was high (63.3/58.7% NRI; 68.3/74.4% OC). At Wk 152, a large proportion of TNFi-n pts had dual achievement of ACR50+PASI100 (46.8% NRI; 60.0% OC). The proportions of TNFi-n pts achieving MDA/VLDA at Wk 152 were 52.1%/31.1% NRI, respectively; 69.0%/41.3% OC, respectively (Table 1; Figure 1).
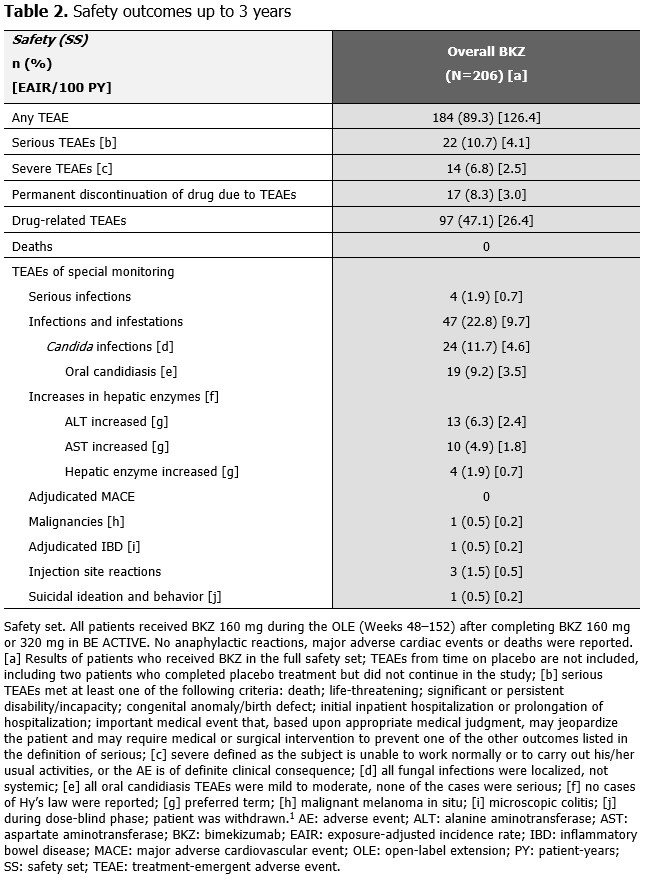
Over 152 wks, the exposure-adjusted incidence rate per 100 pt-years for all BKZ-treated pts was 4.1 for serious TEAEs, 0.7 for serious infections and 4.6 for Candida infections (Table 2). One event was adjudicated by an independent committee as inflammatory bowel disease (microscopic colitis). All Candida infections were localized, mild/moderate and resolved with appropriate antifungal therapy (the majority were oral candidiasis). Overall, 17 pts (8.3%) discontinued study treatment due to TEAEs.
Conclusion: High thresholds of disease control were achieved by >50% of TNFi-n pts treated with BKZ up to 3 years, reflected in long-term improvements in joint and skin outcomes. The safety profile of BKZ up to 3 years in pts with PsA reflects previous observations.1,2
References: 1. Ritchlin CT. Lancet 2020;395:427–40; 2. McInnes IB. Ann Rheum Dis 2020;79:1153–4.
To cite this abstract in AMA style:
Mease P, Deodhar A, Merola J, McInnes I, Assudani D, Bajracharya R, Coarse J, Ink B, Schett G. Bimekizumab in Patients with Psoriatic Arthritis: 3-Year Results for Overall and Tumor Necrosis Factor Inhibitor (TNFi)-Naïve Populations from a Phase 2b Open-Label Extension Study [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/bimekizumab-in-patients-with-psoriatic-arthritis-3-year-results-for-overall-and-tumor-necrosis-factor-inhibitor-tnfi-naive-populations-from-a-phase-2b-open-label-extension-study/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/bimekizumab-in-patients-with-psoriatic-arthritis-3-year-results-for-overall-and-tumor-necrosis-factor-inhibitor-tnfi-naive-populations-from-a-phase-2b-open-label-extension-study/