Session Information
Date: Saturday, November 12, 2022
Title: Abstracts: Spondyloarthritis Including PsA – Treatment I: Axial Spondyloarthritis
Session Type: Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: Bimekizumab (BKZ) is a monoclonal IgG1 antibody that selectively inhibits IL-17F in addition to IL-17A. In patients (pts) with active ankylosing spondylitis (AS), BKZ has demonstrated sustained efficacy and was well tolerated up to 156 weeks (wks) in a phase 2b study, and up to 24 wks in the phase 3 study BE MOBILE 2.1,2,3 Here we present results on the efficacy and safety of BKZ vs placebo (PBO) in pts with active non-radiographic axial spondyloarthritis (nr-axSpA) up to Wk 24 in the ongoing pivotal phase 3 study, BE MOBILE 1.
Methods: BE MOBILE 1 (NCT03928704) comprises a 16-wk double-blind, PBO-controlled period and 36-wk maintenance period. Pts were aged ≥18 years (yrs), had BASDAI ≥4 and spinal pain ≥4 at baseline (BL), and sacroiliitis on MRI and/or elevated CRP at screening. Pts were randomized 1:1 BKZ 160 mg Q4W:PBO. From Wk 16, all pts received BKZ 160 mg Q4W. Primary and secondary efficacy endpoints were assessed at Wk 16, and up to Wk 24. Treatment-emergent adverse events (TEAEs; MedDRA v19.0) are reported among patients who received ≥1 BKZ dose by preferred term up to Wk 24.
Results: Of 254 randomized pts (BKZ: 128; PBO: 126), 244 (96.1%) completed Wk 16, 240 (94.5%) Wk 24. BL characteristics were comparable between groups: mean age 39.4 yrs, symptom duration 9.0 yrs; 45.7% pts were female, 77.6% HLA-B27+ and 10.6% TNFi-inadequate responders (IR).
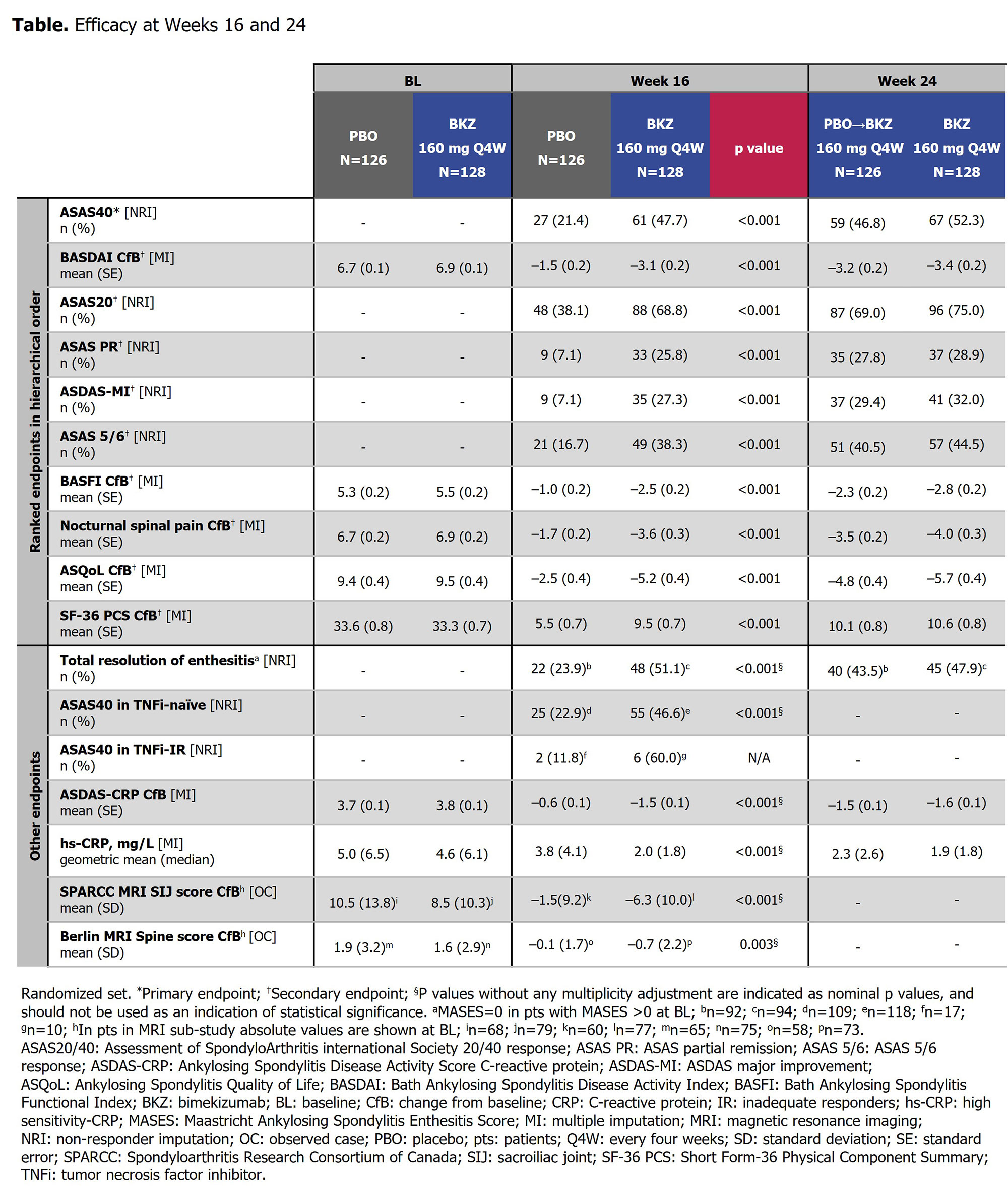
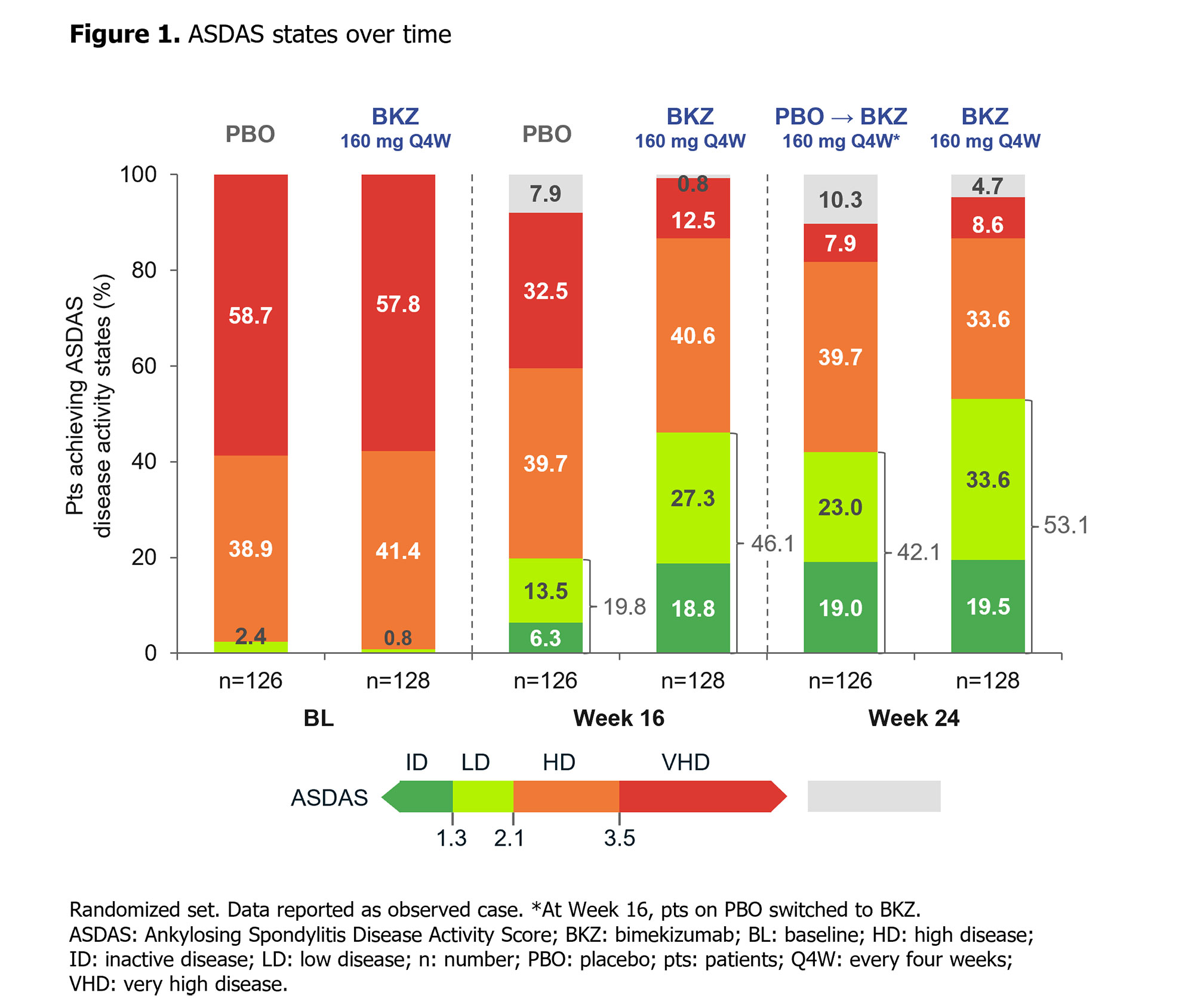
At Wk 16, the primary (ASAS40: 47.7% BKZ vs 21.4% PBO; p< 0.001) and all ranked secondary endpoints were met (Table). ASAS40 responses at Wk 16 were consistent across both TNFi-naïve (46.6% BKZ vs 22.9% PBO) and TNFi-IR (60.0% BKZ vs 11.8% PBO) populations. Responses were rapid with BKZ, including in PBO pts who switched to BKZ at Wk 16, and increased to Wk 24 (Table; Figure 1). At Wk 24, >50% of BKZ-randomized pts achieved ASDAS < 2.1 (Figure 1).
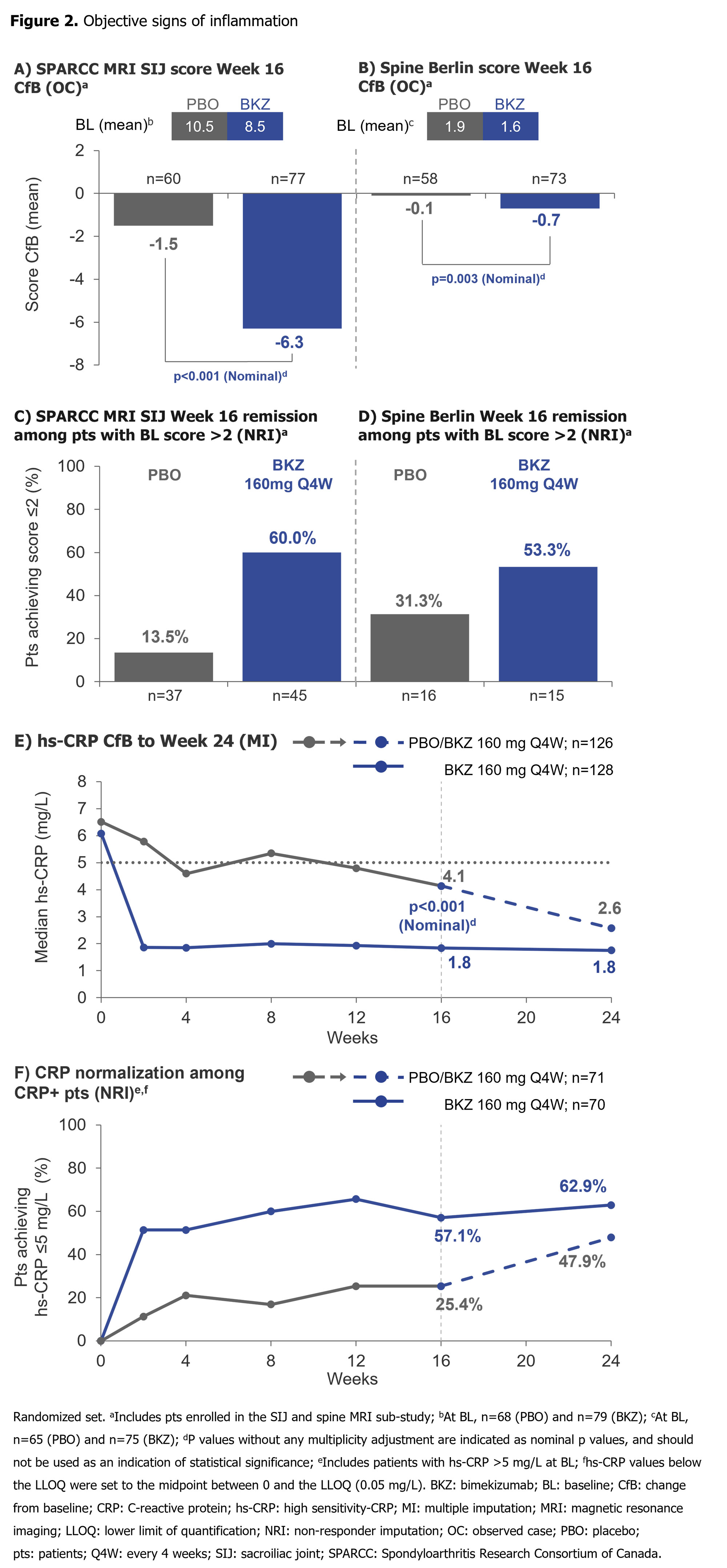
Substantial reductions from BL in SPARCC MRI SIJ inflammation and Berlin MRI Spine scores by Wk 16, and hs-CRP by Wk 2, were achieved with BKZ vs PBO (Figure 2). A higher proportion of pts with SPARCC MRI SIJ and Berlin MRI Spine scores >2 at BL achieved MRI remission (score ≤2) at Wk 16 with BKZ vs PBO. Among CRP+ pts (hs-CRP >5.0 mg/L), a greater proportion treated with BKZ vs PBO achieved normalization (hs-CRP ≤5.0 mg/L) of CRP through Wk 16. For pts who switched from PBO to BKZ at Wk 16, Wk 24 normalization rates approached those seen in BKZ-randomized pts (Figure 2).
Up to Wk 24, 124/244 (50.8%) patients had ≥1 TEAE; most frequent were upper respiratory tract infection (7.0%), nasopharyngitis (6.6%), pharyngitis (2.9%) and oral candidiasis (2.9%). All cases of oral candidiasis were non-severe and non-systemic, and none led to treatment discontinuation. Up to Wk 24, incidence of serious TEAEs was low (0.4%). No IBD, active tuberculosis, MACE or deaths were reported; incidence of uveitis was low (0.8%).
Conclusion: Dual inhibition of IL-17A and IL-17F with BKZ in pts with active nr-axSpA resulted in rapid, clinically relevant improvements in efficacy outcomes vs PBO, including suppression of inflammation. No new safety signals were observed.1,2
References: 1. van der Heijde D. Ann Rheum Dis 2020;79:595–604; 2. Gensler L. Arthritis Rheumatol 2021;73(suppl 10):0491. 3. van der Heijde D. Ann Rheum Dis 2022;OP0019.
To cite this abstract in AMA style:
Deodhar A, van der Heijde D, Gensler L, Xu H, Gaffney K, Dobashi H, Maksymowych W, Rudwaleit M, Magrey M, Elewaut D, Oortgiesen M, Fleurinck C, de Peyrecave N, Ellis A, Vaux T, Shepherd-Smith J, Baraliakos X. Bimekizumab Improves Signs and Symptoms, Including Inflammation, in Patients with Active Non-Radiographic Axial Spondyloarthritis: 24-Week Efficacy & Safety from a Phase 3, Multicenter, Randomized, Placebo Controlled Study [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/bimekizumab-improves-signs-and-symptoms-including-inflammation-in-patients-with-active-non-radiographic-axial-spondyloarthritis-24-week-efficacy-safety-from-a-phase-3-multicenter-randomized-pl/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/bimekizumab-improves-signs-and-symptoms-including-inflammation-in-patients-with-active-non-radiographic-axial-spondyloarthritis-24-week-efficacy-safety-from-a-phase-3-multicenter-randomized-pl/