Session Information
Date: Sunday, November 12, 2023
Title: (0673–0690) Vasculitis – ANCA-Associated Poster I: Treatment Outcomes
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Gucocorticoid (GC)-dependant asthma and ENT exacerbations may persist in more than 80% of eosinophilic granulomatosis with polyangiitis (EGPA). The MIRRA trial demonstrated the efficacy of mepolizumab, a monoclonal antibody targeting interleukin-5 (IL-5), in treating these manifestations. However, roughly 70% of patients still required more than 4 mg per day of prednisone at the end of follow-up or relapsed. Benralizumab, a monoclonal-antibody targeting IL-5 receptor, causes profound eosinophil depletion in plasma, sputum, and tissues. However, its efficacy in GC-dependent manifestations remains to be determined in EGPA. We aimed to describe the use and efficacy of benralizumab in patients with refractory manifestations of EGPA.
Methods: We conducted a multicentre, retrospective study of patients with EGPA according to ACR/EULAR 2022 criteria and treated with benralizumab between February 2019 and February 2023. Complete response was defined as no disease activity (BVAS=0) and a prednisone dose ≤4 mg/day. Partial response was defined as no disease activity and a prednisone dose ≥4 mg/day. Total response was defined by either complete or partial response. Comparisons between patients with and without prior mepolizumab therapy were made using the Fisher exact test and the Mann Whitney test. Comparisons of responses or time to vasculitis flares were made with log-rank test.
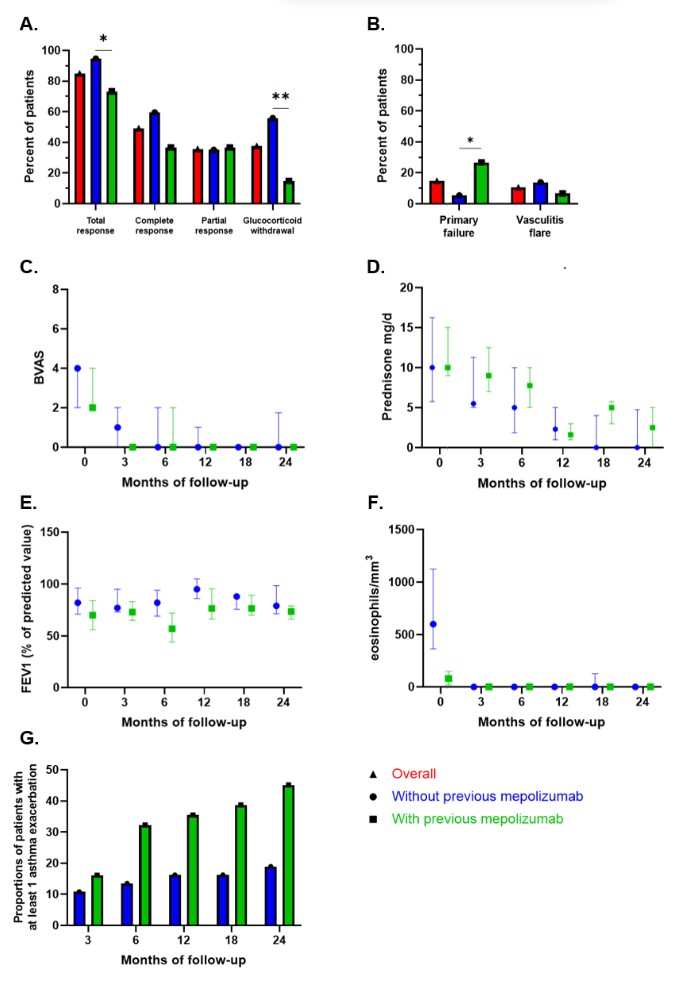
Results: Sixty-eight patients were included. The median age was 50 (IQR 39-63) years. ANCA were positive in 20 (29%) patients at EGPA diagnosis. Thirty-one patients (46%) had previously received mepolizumab, at a dose of 100 mg/month in 18/26 (69%) and 300 mg/month in 8/26 (31%). Primary or secondary failure of mepolizumab accounted for 16 patients (52%) and 15 patients (48%). The use of benralizumab was justified by uncontrolled asthma in 54 (81%), uncontrolled ENT manifestations in 27 (40%) and persistent glucocorticoid use in 48 (74%) patients. The asthma regimen (30 mg/month 3 times then every 2 months) was used in 63/66 patients (95%). Sixteen patients (24%) were concomitantly treated with another immunosuppressant.
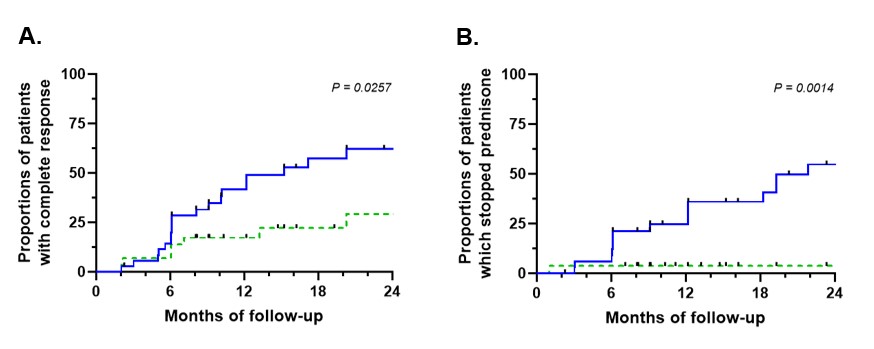
Median follow-up after initiation of benralizumab was 23 months (IQR 9-34). Thirty-three patients (49%) had a complete response, 24 (36%) had a partial response and 10 (15%) failed to respond (response assessed in 67 patients). Of 57 patients with at least a partial initial response, 10 (18%) experienced secondary failure. GCs were discontinued in 23 patients (38%). Prior mepolizumab use was associated with more primary failure (p=0.034) and less GC discontinuation (p=0.001).
Vasculitis flares occurred in 7 patients (11%) and were associated with histological evidence of vasculitis and/or ANCA positivity at benralizumab initiation (p=0.010). Six infections requiring specific treatments were reported during follow-up.
Conclusion: Benralizumab appears to be an effective treatment for refractory asthma or ENT manifestations in EGPA and allows glucocorticoid withdrawal. However, its efficacy was lower after prior mepolizumab failure.
IS = immunosuppressant; * = P < 0.05; ** = P < 0.01 (Fisher exact test).
To cite this abstract in AMA style:
Cottu A, Groh M, Desaintjean C, Marchand-Adam S, Guillevin L, Puéchal X, Lazaro E, Samson M, Taillé C, Durel C, Diot E, Nicolas S, Guilleminault L, Ebbo M, Cathébras P, Dupin C, Yildiz H, Belfeki N, Pugnet G, Chauvin P, Jouneau S, Lifermann F, Martellosio J, Cottin V, Terrier B. Benralizumab in Eosinophilic Granulomatosis with Polyangiitis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/benralizumab-in-eosinophilic-granulomatosis-with-polyangiitis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/benralizumab-in-eosinophilic-granulomatosis-with-polyangiitis/