Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Treatment for psoriatic arthritis (PsA) often involves biologic and targeted synthetic DMARDs (b/tsDMARDs). Existing research suggests that starting treatment with b/tsDMARDs early may lead to greater clinical responses, although few examine whether early response to treatment yields even greater clinical improvements. This study aims to evaluate and compare clinical and patient-reported outcomes (PROs) observed in patients with PsA who respond early versus late to b/tsDMARD treatment in a real-world setting.
Methods: This analysis evaluated data from the CorEvitas PsA/Spondyloarthritis Registry, a prospective, multicenter, noninterventional registry. Adult patients who achieved minimal disease activity (MDA) at 6 months and maintained at 12 months (Early Sustained MDA) were compared with those who did not achieve MDA at 6 months but achieved MDA at 12 months (Late MDA). Modified Poisson and linear regression models were used to estimate the association between MDA groups (Early Sustained vs Late) and outcomes at 12-month follow-up. Adjusted relative risks (RR; 95% confidence interval [CI]) and β coefficients (95% CI) were used to assess disease activity measures and PROs.
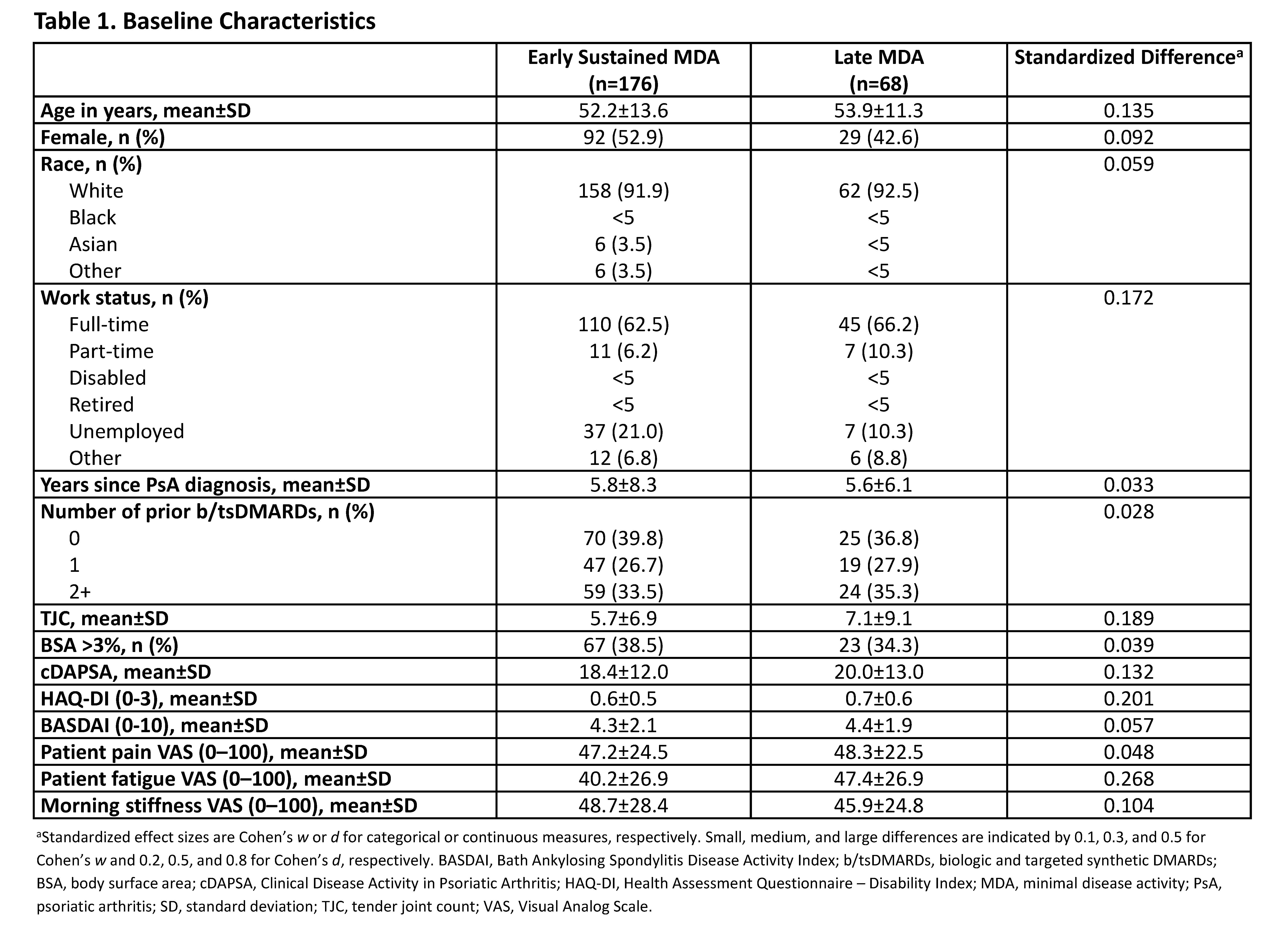
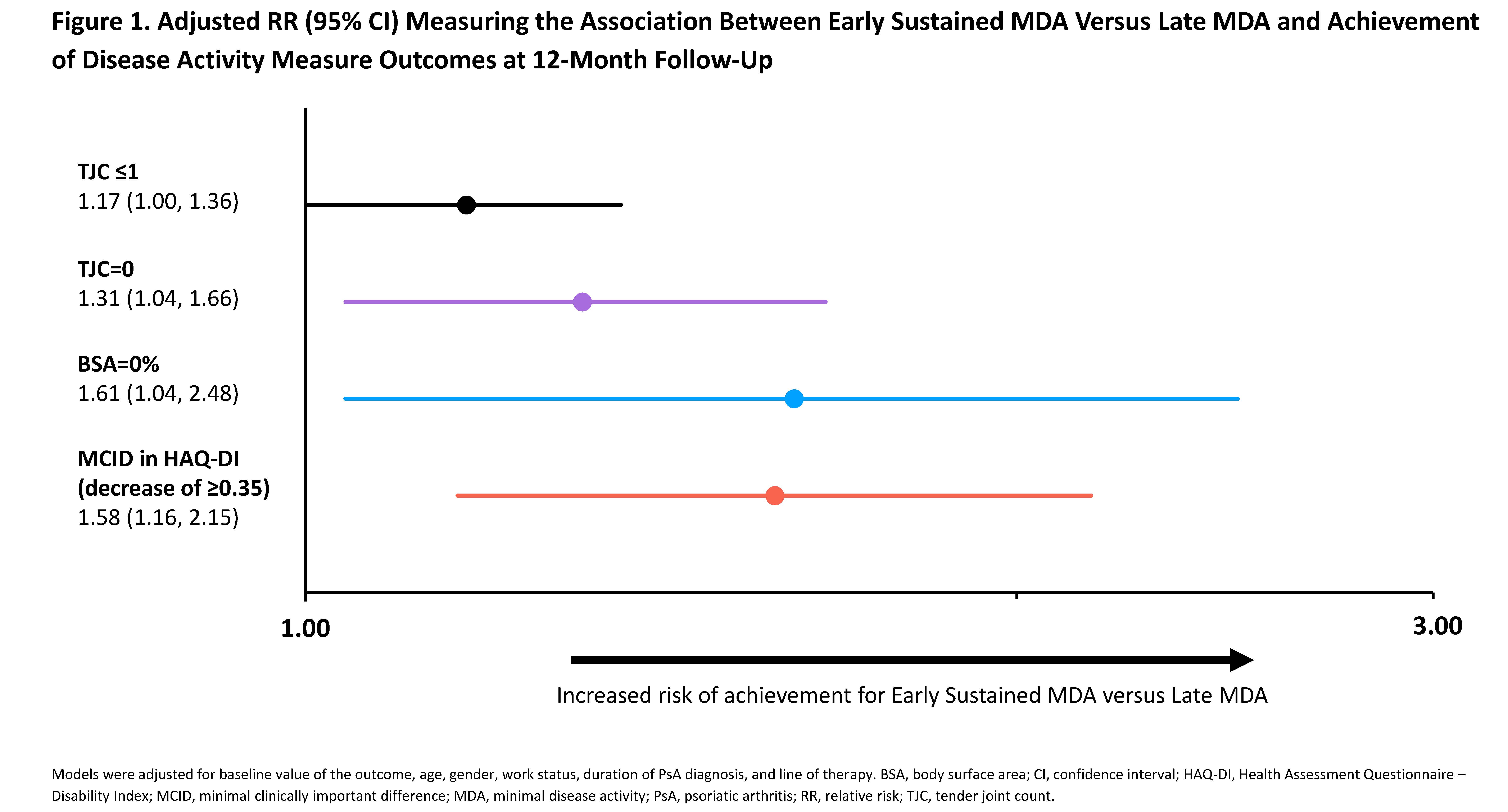
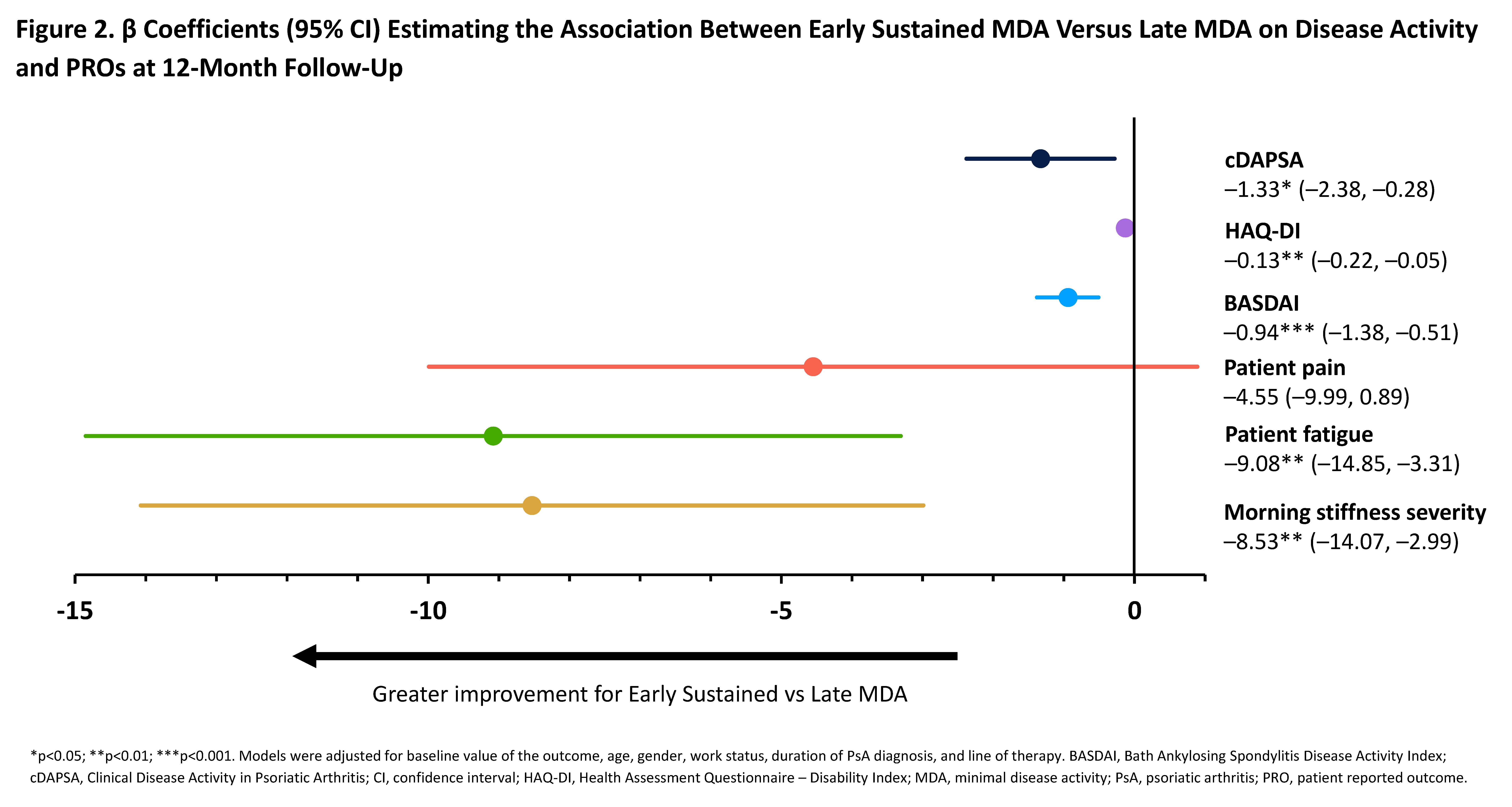
Results: This analysis (N=244) identified 176 “Early Sustained MDA” and 68 “Late MDA” patients (Table 1). Baseline characteristics were similar between groups, except for tender joint count (TJC), Health Assessment Questionnaire – Disability Index (HAQ-DI), and patient fatigue, which were higher among the Late versus Early Sustained MDA group. At 12-month follow-up, Early Sustained MDA patients achieved low disease activity levels and improvements in PROs at a higher rate (RR >1) than Late MDA patients: TJC ≤1 (RR: 1.17 [95% CI: 1.00, 1.36]) and TJC=0 (1.31 [1.04, 1.66]), body surface area (BSA)=0% (1.61 [1.04, 2.48]), and minimal clinically important difference (MCID) in HAQ-DI (defined as decrease of ≥0.35; 1.58 [1.16, 2.15]) (Figure 1). Significantly greater improvements were observed at 12-month follow-up in Clinical Disease Activity in Psoriatic Arthritis (cDAPSA), HAQ-DI, Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), patient fatigue, and morning stiffness in Early Sustained compared with Late MDA achievers (Figure 2). Although greater improvements in pain were observed in Early Sustained achievers compared to Late MDA achievers at 12-month follow-up, this result was not statistically significant.
Conclusion: Patients with PsA who achieved MDA early and sustained stringent disease control demonstrated greater improvements in disease activity and PROs compared with those who achieved MDA late. These findings demonstrate the value of achieving early and sustained disease control for optimal patient outcomes.
To cite this abstract in AMA style:
Mease P, Ye X, Saffore C, Iyile T, Magalhaes Reis Nakasato P, Blachley T, Lakin P, Middaugh N, Ogdie A. Benefits of Achieving Early versus Late Clinical Response After Treatment with Biologic and Targeted Synthetic DMARDs Among Patients with PsA in the CorEvitas PsA/Spondyloarthritis Registry [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/benefits-of-achieving-early-versus-late-clinical-response-after-treatment-with-biologic-and-targeted-synthetic-dmards-among-patients-with-psa-in-the-corevitas-psa-spondyloarthritis-registry/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/benefits-of-achieving-early-versus-late-clinical-response-after-treatment-with-biologic-and-targeted-synthetic-dmards-among-patients-with-psa-in-the-corevitas-psa-spondyloarthritis-registry/