Session Information
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: SB4, a biosimilar to the reference ETN, received EU marketing authorisation in January 2016, based on the totality of evidence from pre-clinical and clinical Phase I and III studies that demonstrated similar efficacy, bioequivalence, and comparable safety and immunogenicity to ETN. The BENEFIT study describes real world evidence on outcomes of transition from originator to biosimilar in both rheumatoid arthritis (RA) and axial spondyloarthritis (axSpA) in 4 EU countries, outside the controlled setting of a randomised clinical trial, with the purpose of providing real world evidence on transition and outcomes of treatment switch in routine clinical practice.
Methods: Eligible patients had RA or axSpA and had initiated SB4 following treatment with a stable dose of originator ETN in a 6-month window. Data were captured from clinic records, retrospectively for 6 months prior and prospectively and/or retrospectively for 6 months after switch. Outcomes include disease score (DAS-28 for RA, BASDAI for axSpA) over time, clinical characteristics and management, and serious adverse events (SAEs).
Results: Of the 557 patients included, 358 had RA and 199 axSpA; data at baseline and after transition are illustrated in Table 1.
One adverse event of pneumonia was reported as Serious (SAE) and related to SB4. Six SAEs unrelated to SB4 were reported: Uveitis, umbilical hernia, relapsing pancreatitis, coronary artery disease, chronic obstructive pulmonary disease and lithium overdose.
Conclusion: These data from clinical practice indicate maintenance of disease status post-switch from ETN to SB4, without the need for dose adjustment, and with high persistence at 6 months after switch, in both RA and axSpA patients. No safety concerns were observed. The study data provide pertinent information about 6-month outcomes in these populations, helping to inform evidence-based treatment decisions.
Acknowledgements:
Biogen International GmbH funded and sponsored this study. Authors had full editorial control and provided final approval of all content.
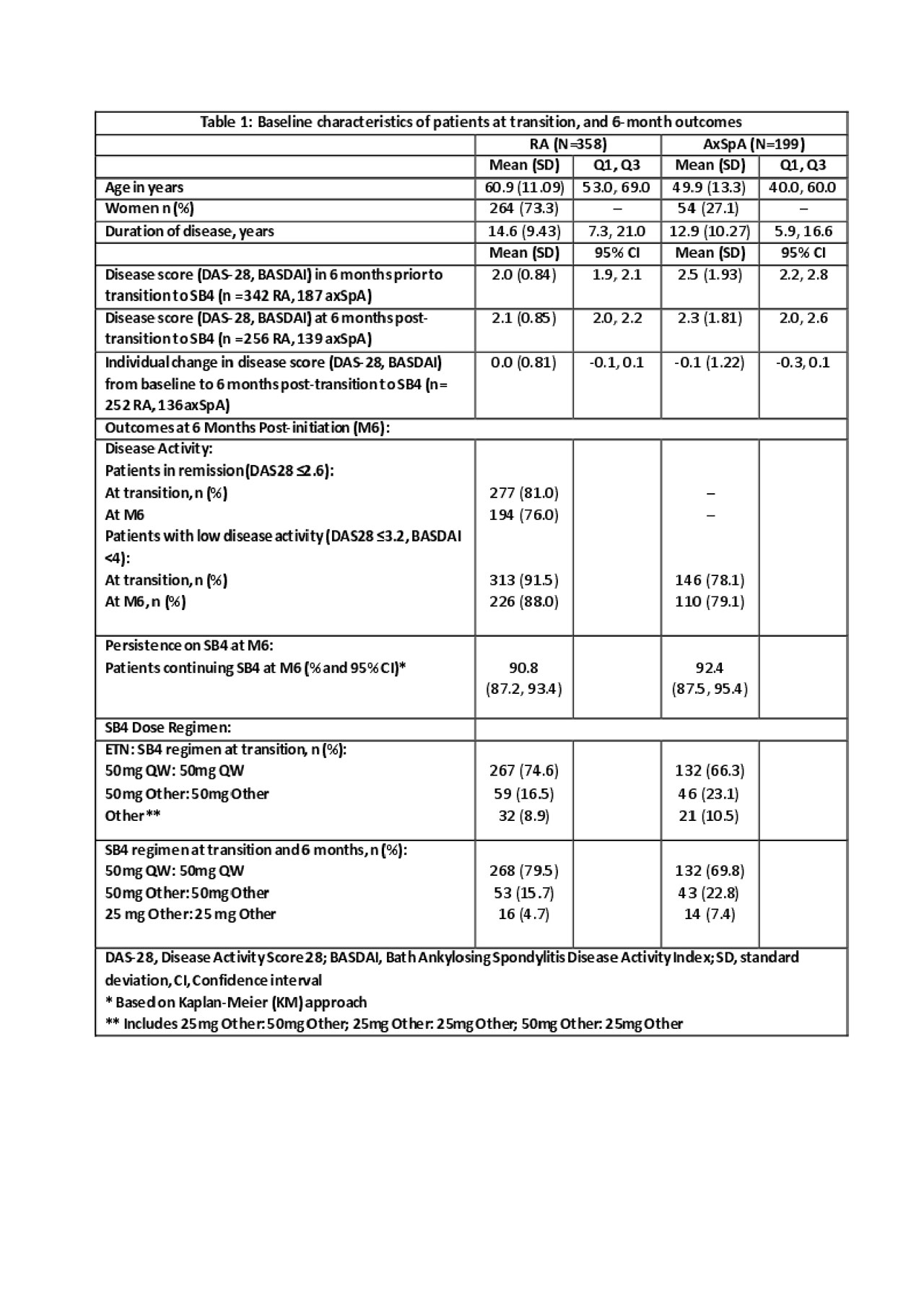
Table 1 -to be placed following first paragraph of Results section-
To cite this abstract in AMA style:
Selmi C, Krüger K, Cantagrel A, Hernández A, Freudensprung U, Rezk M, Addison J. ‘BENEFIT’ Pan-European Observational Study to Evaluate the Real-world Effectiveness of SB4 Transition from Originator Etanercept (ETN) in Patients with Rheumatoid Arthritis or Axial Spondyloarthritis: A Switch Success Story [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/benefit-pan-european-observational-study-to-evaluate-the-real-world-effectiveness-of-sb4-transition-from-originator-etanercept-etn-in-patients-with-rheumatoid-arthritis-or-axial-sp/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/benefit-pan-european-observational-study-to-evaluate-the-real-world-effectiveness-of-sb4-transition-from-originator-etanercept-etn-in-patients-with-rheumatoid-arthritis-or-axial-sp/
