Session Information
Date: Sunday, November 17, 2024
Title: SLE – Treatment Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
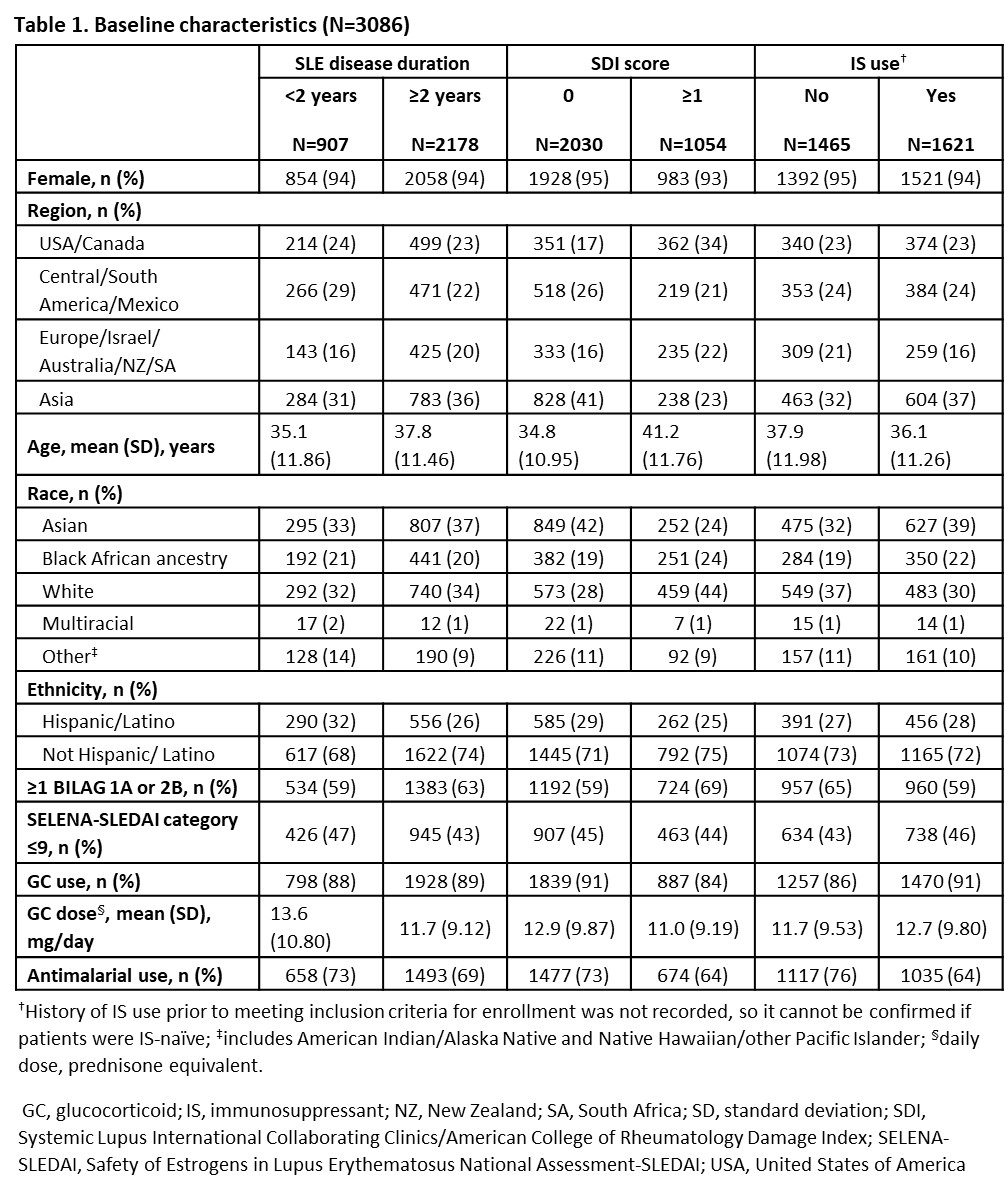
Background/Purpose: There are benefits of early treatment for autoimmune diseases; however, data are not available for patients (pts) with SLE as there is no definition of early disease (EYD). Updated European Alliance of Associations for Rheumatology recommendations for SLE management consider biologics in pts unresponsive to HCQ (+/- glucocorticoids [GC]) or pts unable to reduce GCs.1 In advance of robust, prospective data evaluating earlier biologic use, pooled analyses of belimumab (BEL) data from registrational clinical trials may provide insights. This analysis used data from BEL clinical trials to evaluate BEL vs placebo (PBO) plus standard therapy (ST) on SLE Responder Index (SRI)-4 in pts with active SLE across EYD and established disease (ESD) subgroups, defined based on baseline SLE disease duration (< 2 or ≥2 years), baseline Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index (SDI) score (0 or ≥1) and baseline immunosuppressant (IS) use (no or yes).
Methods: Integrated data from Phase 3 BEL trials in adults with active SLE, from ~10 years (BLISS-76, BLISS-52, North East Asia, EMBRACE, and BLISS-SC) was used in this post hoc analysis.2–6 Included pts were randomized to BEL (10 mg/kg/month intravenously or 200 mg/week subcutaneously) or PBO, plus ST. SRI-4 responses at Week 52 with BEL vs PBO, plus ST, were evaluated in EYD (baseline SLE disease duration < 2 years, SDI=0, no IS use) and ESD (baseline SLE disease duration ≥2 years, SDI≥1, IS use). Logistic regression models estimated odds ratios (OR), 95% confidence intervals (CI) and p values for BEL vs PBO, adjusting for study, and baseline Safety of Estrogens in Lupus Erythematosus National Assessment (SELENA)–SLEDAI score (≤9 vs ≥10).
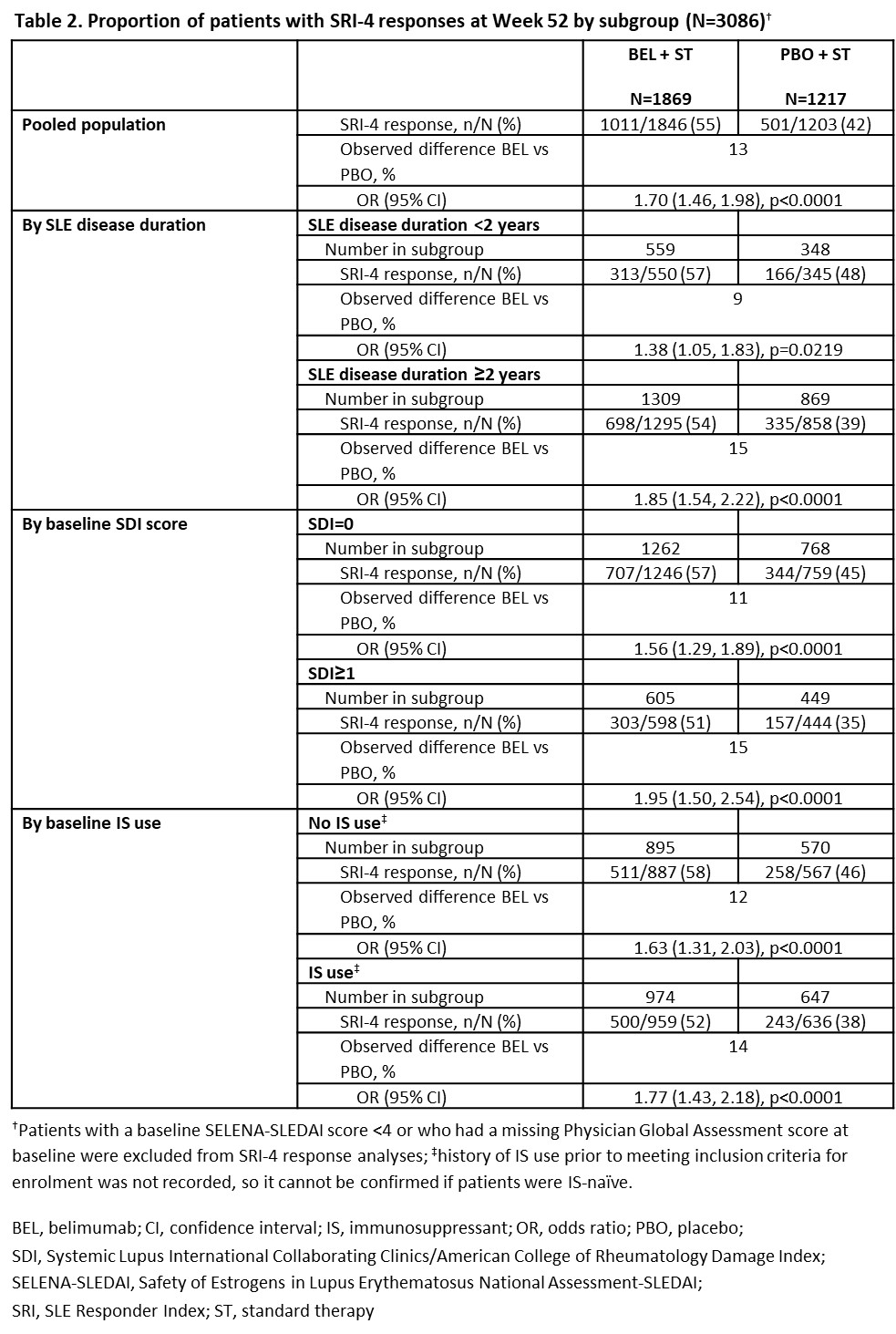
Results: 3086 pts (BEL, n=1869; PBO, n=1217) were included. Differences in baseline characteristics were observed (Table 1). More pts achieved SRI-4 response at Week 52 with BEL vs PBO in the pooled population (OR [95% CI]: 1.70 [1.46, 1.98], p< 0.0001), and regardless of baseline SLE disease duration (< 2 years: 1.38 [1.05, 1.83], p=0.0219; ≥2 years: 1.85 [1.54, 2.22], p< 0.0001), SDI score at baseline (SDI=0: 1.56 [1.29, 1.89]; SDI ≥1: 1.95 [1.50, 2.54]; both p< 0.0001) or baseline IS use (no: 1.63 [1.31, 2.03]; yes: 1.77 [1.43, 2.18]; both p< 0.0001). The proportion of SRI-4 responders was higher in all EYD subgroups for both BEL (57–58%) and PBO (45–48%), compared with the pooled population (BEL, 55%; PBO, 42%) and ESD subgroups (BEL, 51–54%; PBO, 35–39%; Table 2).
Conclusion: This post hoc analysis confirmed the efficacy of BEL vs PBO across EYD and ESD subgroups. Pts with EYD had higher responses than those with ESD, but smaller treatment differences. These trials were not designed to evaluate SRI-4 response in an EYD population; however, data support use of BEL in EYD to improve outcomes. Longer studies in pts with EYD are needed to confirm these findings.
References
1. Fanouriakis A et al. Ann Rheum Dis. 2024;83:15-29
2. Navarra SV et al. Lancet. 2011;377:721-31
3. Furie R et al. Arthritis Rheum. 2011;63:3918-30
4. Zhang F et al. Ann Rheum Dis. 2018;77:355-63
5. Stohl W et al. Arthritis Rheum. 2017;69:1016-27
6. Ginzler E et al. Arthritis Rheum. 2022;74:112-23
Disclosures: K. Costenbader: AstraZeneca, 2, BMS, 2, Cabaletta Bio, 2, Exagen Diagnostics, 5, Gilead, 5, GSK, 2, Merck, 5; J. Merrill: AbbVie, 2, 6, 12, Instructor fees, Amgen, 2, AstraZeneca, 2, 5, 6, Aurinia, 2, 6, Bristol-Myers Squibb(BMS), 2, 5, 6, 12, Instructor fees, EMD Serono, 2, Genentech, 2, Gilead, 2, GSK, 2, 5, 6, Kezar, 2, Lilly, 2, Merck/MSD, 2, Pfizer, 2, RemeGen, 2, 12, Instructor fees, Sanofi/Provention, 2, 12, Instructor fees, Takeda, 2, 12, Instructor fees, UCB, 2, Xencor, 2, 12, Instructor fees, Zenas, 2; M. Mosca: AbbVie, 2, 6, AstraZeneca, 2, 6, GSK, 2, 5, 6, Idorsia, 2, Janssen, 6, Lilly, 6, Otsuka, 2, 6, UCB, 2; H. Quasny: GSK, 2, 7; C. Henning: GSK, 3, 11; S. Bloom: GSK, 2, 7; J. Harris: GSK, 3, 11; C. O’Shea: GSK, 3, 11; T. Atsumi: AbbVie, 6, Alexion Inc., 6, Asahi-Kasei Co., 6, Astellas Pharma Inc., 6, AstraZeneca, 2, 6, Bayer Yakuhin, 6, Bristol-Myers Squibb(BMS), 6, Chugai Pharmaceutical Co., Ltd., 6, Daiichi Sankyo Co., Ltd., 6, Eisai Co. Ltd., 6, Eli Lilly Japan K.K., 6, Gilead Sciences K.K., 6, GSK, 2, 5, Janssen, 6, Mitsubishi Tanabe Pharma Co., 6, Nippon Boehringer Ingelheim Co., Ltd., 2, 6, Nippon Shinyaku Co., Ltd., 6, Novartis, 2, 6, Otsuka, 2, Pfizer, 6, Taiho Pharmaceutical Co. Ltd., 6, UCB, 6; R. van Vollenhoven: AbbVie, 2, 6, AstraZeneca, 2, 6, 12, Instructor fees, Biogen, 2, Bristol-Myers Squibb(BMS), 2, 5, 6, Galapagos, 2, 6, 12, Instructor fees, GSK, 2, 6, Janssen, 2, 6, Merck/MSD, 12, Instructor fees, Novartis, 12, Instructor fees, Pfizer, 2, 6, 12, Instructor fees, RemeGen, 2, Roche, 12, Instructor fees, Sanofi, 12, Instructor fees, UCB, 2, 5, 6, 12, Instructor fees.
To cite this abstract in AMA style:
Costenbader K, Merrill J, Mosca M, Quasny H, Henning C, Bloom S, Harris J, O’Shea C, Atsumi T, van Vollenhoven R. Belimumab Increases SLE Responder Index-4 Response Rates versus Placebo in Early Active Systemic Lupus Erythematosus: A Large Integrated Analysis of Belimumab Trials [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/belimumab-increases-sle-responder-index-4-response-rates-versus-placebo-in-early-active-systemic-lupus-erythematosus-a-large-integrated-analysis-of-belimumab-trials/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/belimumab-increases-sle-responder-index-4-response-rates-versus-placebo-in-early-active-systemic-lupus-erythematosus-a-large-integrated-analysis-of-belimumab-trials/