Session Information
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: We report the baseline characteristics of a large cohort of diffuse cutaneous systemic sclerosis (dcSSc) patients enrolled in a Phase 3 trial of lenabasum, a preferential cannabinoid receptor type 2 agonist. Treatment with lenabasum, a cannabinoid receptor type 2 agonist, was safe and well-tolerated in a prior Phase 2 study in dcSSc patients and associated with improvements in ACR Combined Response Index in diffuse cutaneous Systemic Sclerosis (CRISS) score and multiple secondary efficacy outcomes.
Methods: The RESOLVE-1 Phase 3 study was designed with input from Principal Investigators, other study investigators, and regulatory authorities in the US, EU, and Japan. An important intent of the design was to have eligibility criteria that allow testing of efficacy and safety of lenabasum in an inclusive group of dcSSc subjects to maximize relevance to patients in current practice. The study is ongoing and remains blinded.
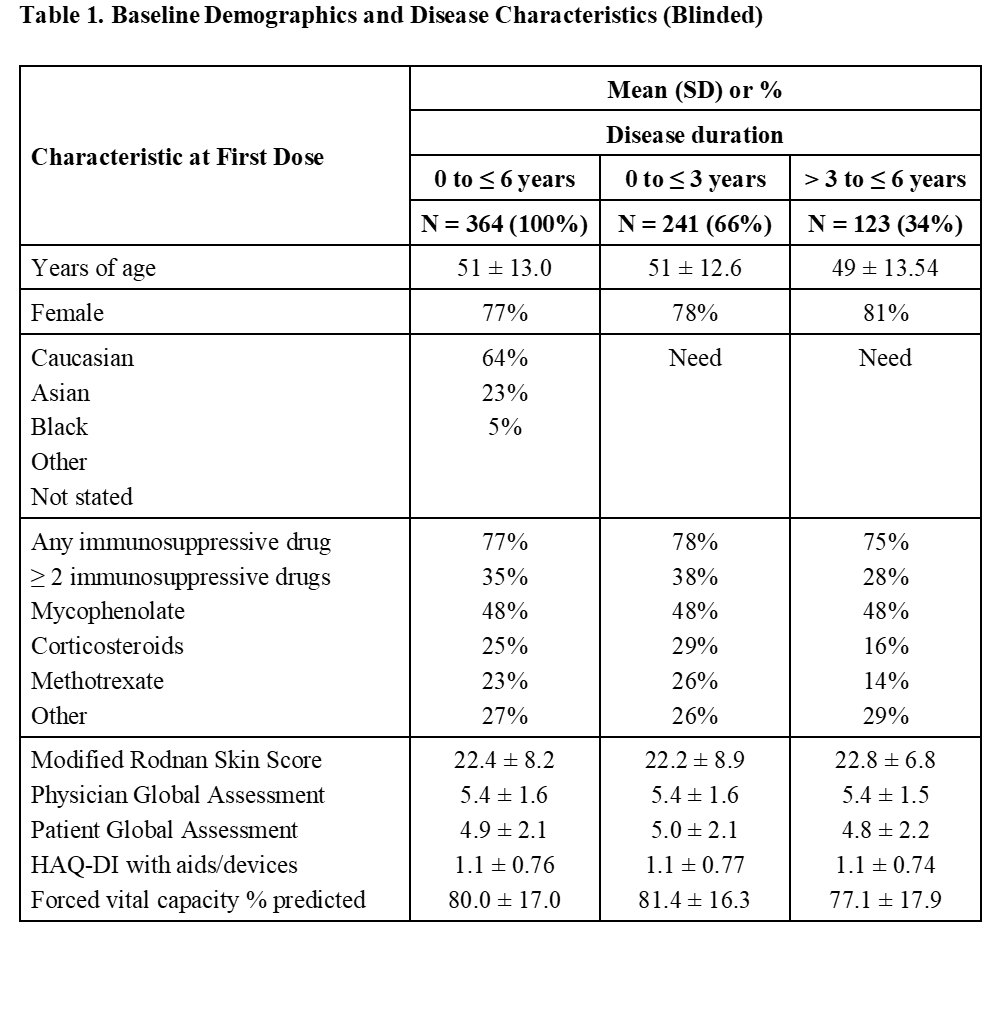
Results: Primary efficacy outcome is the ACR CRISS score at 12 months, comparing lenabasum 20 mg BID to placebo. Key inclusion criteria are males and females ≥ 18 years of age with dcSSc and disease duration ≤ 6 years who are on stable standard of care medicines, with immunosuppressive mediations allowed. Exceptions are concomitant treatment with > 10 mg per day prednisone or equivalent is disallowed and mRSS needs to be ≥ 15 if disease duration is > 3 to ≤ 6 years. The study enrolled 364 subjects over 15 months who received ≥ 1 dose of study drug at 77 sites in 13 countries in North America (n = 139), Europe (n = 109), and Israel (n = 37), and Asia-Pacific (n = 79), with last subject first visit on May 1, 2019. Baseline characteristics as shown in Table 1. The majority were middle-aged, female, and white, and 77% were on immunosuppressive drugs. Mycophenolate/mycophenolic acid used in 48% of subjects, and 35% of subjects took ≥ 2 concurrent immunosuppressive drugs (max = 4 concurrent). Subjects with disease duration ≤ 3 years and > 3 to ≤ 6 years had similar demographics and disease characteristics, except a lower proportion of the subjects with longer disease duration were on methotrexate (p = 0.041, Chi-square), low dose corticosteroids (p = 0.006, Chi-square), or multiple immunosuppressive medications (p = 0.055, Chi-square). Subjects with longer disease duration also had slightly lower FVC % predicted (p = 0.018, t-test).
Conclusion: This is the first Phase 3 study to use ACR CRISS as the primary efficacy outcome, a composite outcome of multiple clinically relevant measures of SSc, and the largest interventional study to date in diffuse cutaneous SSc. Benefits of having inclusive eligibility criteria are that they facilitated timely full enrollment and will make the study population representative of real-world practice, if trial is positive. This study provides a template for Phase 3 dcSSc trials and will give valuable information on outcome with routine care as well as test efficacy of lenabasum.
To cite this abstract in AMA style:
Spiera R, Dgetluck N, Bloom B, White B, Denton C. Baseline Subject Demographics and Disease Characteristics in a Phase 3 Study of Safety and Efficacy of Lenabasum in Diffuse Cutaneous Systemic Sclerosis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/baseline-subject-demographics-and-disease-characteristics-in-a-phase-3-study-of-safety-and-efficacy-of-lenabasum-in-diffuse-cutaneous-systemic-sclerosis/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/baseline-subject-demographics-and-disease-characteristics-in-a-phase-3-study-of-safety-and-efficacy-of-lenabasum-in-diffuse-cutaneous-systemic-sclerosis/