Session Information
Date: Monday, November 9, 2020
Session Type: Abstract Session
Session Time: 12:00PM-12:50PM
Background/Purpose: Radiographic spinal progression determinates functional status and mobility in ankylosing spondylitis (AS) (Poddubnyy et al, 2018). Our objective was to analyze whether biomarker levels at baseline or their change after 3 months or 2 years can predict spinal radiographic progression in AS patients treated with TNF-α inhibitors (TNFi).
Methods: Consecutive AS patients from the Groningen Leeuwarden Axial Spondyloarthritis (GLAS) cohort (Maas et al, 2019) starting TNFi between 2004 and 2012 were included. The following serum biomarkers were measured at baseline, 3 months and 2 years of follow-up with commercially available ELISA: calprotectin, matrix metalloproteinase-3 (MMP-3), serum amyloid A (SAA), vascular endothelial growth factor (VEGF), osteoprotegerin (OPG), procollagen type II N-terminal propeptide (PIINP), sclerostin, high molecular weight adiponectin, leptin and visfatin. Two independent readers assessed spinal radiographs according to the modified Stoke Ankylosing Spondylitis Spine Score (mSASSS).
Logistic regression was performed to examine the association between biomarker values at baseline, their change after 3 months and 2 years and radiographic spinal progression (defined as mSASSS change ≥2 units or the formation of ≥1 new syndesmophyte over 2 years). Multivariable models for each biomarker were adjusted for mSASSS or syndesmophytes at baseline, elevated CRP (≥5mg/l), smoking status, male gender, symptom duration, BMI, and baseline biomarker level (the latter only in models with biomarker change).
Results: Of the 137 included AS patients, 72% were male, 79% HLAb27+, mean age was 42 years (SD 10.8) and baseline ASDAScrp 3.8 (SD 0.8). Serum levels of calprotectin, MMP-3, SAA and VEGF showed a significant reduction after 3 months and 2 years of TNFi therapy. OPG and PIINP decreased significantly after 3 months, but did not differ from baseline after 2 years. Sclerostin increased after 2 years.
Univariable logistic regression revealed a significant association of baseline visfatin (odds ratio (OR) [95% confidence interval] 1.106 [1.007-1.215]) and sclerostin serum levels (OR 1.006 [1.001-1.011]) with radiographic progression defined as mSASSS change ≥2 units after 2 years. In multivariable logistic analysis, only baseline visfatin level remained significantly associated (OR 1.337 [1.088-1.642]). Furthermore, baseline calprotectin showed a positive association (OR 1.146 [1,011-1,299]) when adjusting for known risk factors for radiographic spinal progression. Interestingly, the change of visfatin level after 2 years was associated with both measures of radiographic progression after 2 years – mSASSS progression (OR 1.108 [1.004-1.224]) and syndesmophyte formation (OR 1.115; [1.002-1.24]). However, those associations were lost in multivariate analysis.
Conclusion: While serum levels of biomarkers of inflammation (calprotectin, MMP-3, SAA, VEGF) and bone formation (OPG, PIINP, sclerostin) showed significant changes under TNFi therapy, adipokine levels (HMW-adiponektin, leptin, visfatin) were not altered. Independent of known risk factors, baseline calprotectin and visfatin levels were associated with radiographic spinal progression after 2 years of TNFi.
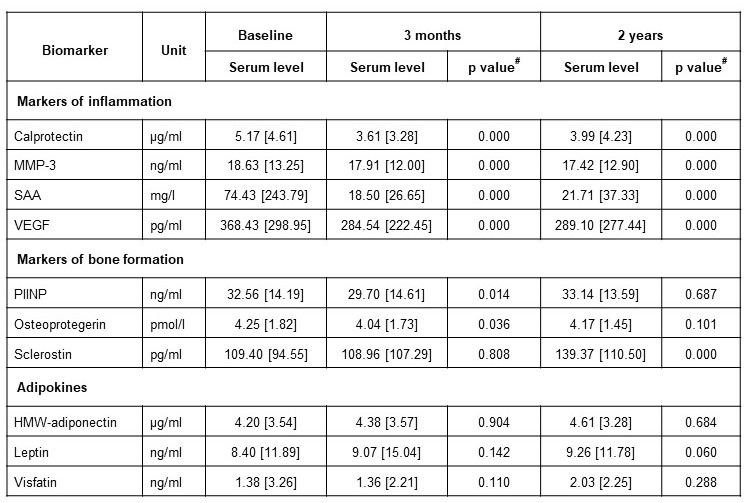 Table 1. Serum biomarker levels at baseline (before start of TNFi), after 3 months and 2 years. Values presented as medians [interquartile range (IQR)]. # Wilcoxon signed-rank test compared to baseline. HMW high molecular weight. MMP-3 matrix metalloproteinase-3. PIINP procollagen type II N-terminal propeptide. SAA serum amyloid A. VEGF vascular endothelial growth factor.
Table 1. Serum biomarker levels at baseline (before start of TNFi), after 3 months and 2 years. Values presented as medians [interquartile range (IQR)]. # Wilcoxon signed-rank test compared to baseline. HMW high molecular weight. MMP-3 matrix metalloproteinase-3. PIINP procollagen type II N-terminal propeptide. SAA serum amyloid A. VEGF vascular endothelial growth factor.
To cite this abstract in AMA style:
Rademacher J, Siderius M, Gellert L, Wink F, Verba M, Maas F, Tietz L, Poddubnyy D, Spoorenberg A, Arends S. Baseline Serum Biomarker Levels Predict Spinal Radiographic Progression in Ankylosing Spondylitis Patients on TNF Inhibitor Therapy [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/baseline-serum-biomarker-levels-predict-spinal-radiographic-progression-in-ankylosing-spondylitis-patients-on-tnf-inhibitor-therapy/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/baseline-serum-biomarker-levels-predict-spinal-radiographic-progression-in-ankylosing-spondylitis-patients-on-tnf-inhibitor-therapy/
