Session Information
Date: Sunday, October 26, 2025
Session Type: Abstract Session
Session Time: 1:45PM-2:00PM
Background/Purpose: MAS is a life-threatening complication of Still’s disease, characterized by IFNg-driven macrophage activation and systemic hyperinflammation. Chemokine C-X-C motif ligand 9 (CXCL9) is released in response to IFNg and can be used as a marker of IFNg activity. Emapalumab binds free and receptor-bound IFNg, providing rapid and targeted neutralization of IFNg. Emapalumab achieved rapid control of MAS in a pooled patient population from two open-label, single-arm interventional studies (NI-0501-06 [NCT03311854] and NI-0501-14 [EMERALD; NCT05001737]). Changes in key PD markers in this population were evaluated according to achievement of response and time to response.
Methods: Patients with MAS in Still’s disease who had an inadequate response to high-dose glucocorticoids (GCs), or investigator-assessed rapid worsening of clinical condition and/or laboratory parameters, were treated with emapalumab for 4 weeks: a 6 mg/kg loading dose, followed by 3 mg/kg every 3 days from days 4–16, then 3 mg/kg twice weekly until Day 28 (or longer if insufficient clinical response). The primary efficacy endpoint was complete response (CR) at Week 8, defined as resolution of clinical signs according to investigator assessment (visual analog scale [VAS] ≤1/10 cm) and normalization of 7 MAS-related laboratory parameters. Overall response (OR) was defined as a CR or partial response (PR; VAS < 4 cm and normalization of ≥3 abnormal baseline laboratory parameters included in the composite primary endpoint).
Results: 39 patients were enrolled (31 [79.5%] females; median age, 12 years [range, 9 months–64 years]). At Week 8, 21/39 (53.8%) patients treated with emapalumab achieved a CR (95% confidence interval, 37.2–69.9%) and 29/38 patients (76.3%) had an OR at Week 8. The median time to first OR was 16 days with 32/39 patients achieving an OR before Week 8 and 6 patients had their first response after Week 8. (Figure 1). Once an OR was reached, response was generally maintained through Week 8 and most patients were able to reach CR (Figure 2). Patients with CR (n=20) or PR (n=9) at Week 8 had higher geometric mean levels of CXCL9 (CR, 3206 ng/mL; PR, 2371 ng/mL), ferritin (CR, 8186 μg/mL; PR, 8452 μg/mL) and soluble CD25 (sCD25; CR, 5654 ng/mL; PR, 7436 ng/mL) at baseline versus Week 8 non-responders (n=5) (CXCL9, 291 ng/mL; ferritin, 2633 μg/mL; sCD25, 1531 ng/mL).All patients had rapid and sustained CXCL9 and ferritin declines over time: at Week 8, CXCL9 and ferritin median percentage reductions from baseline were -99% and -92% in patients with CR or PR, respectively. NR had similar declines in CXCL9 (n=2; -86%) and ferritin (n=5; -89%) at Week 8, though sCD25 remained relatively unchanged vs baseline (n=2; +21%).
Conclusion: Half of the patients with MAS secondary to Still’s disease with an inadequate response to high-dose GCs treated with emapalumab achieved a first OR within 16 days and most patients having a sustained treatment effect reaching a CR at Week 8. Emapalumab treatment rapidly reduced key PD markers, including CXCL9 and ferritin, aligning with IFNγ’s role in MAS and emapalumab’s mechanism of action.
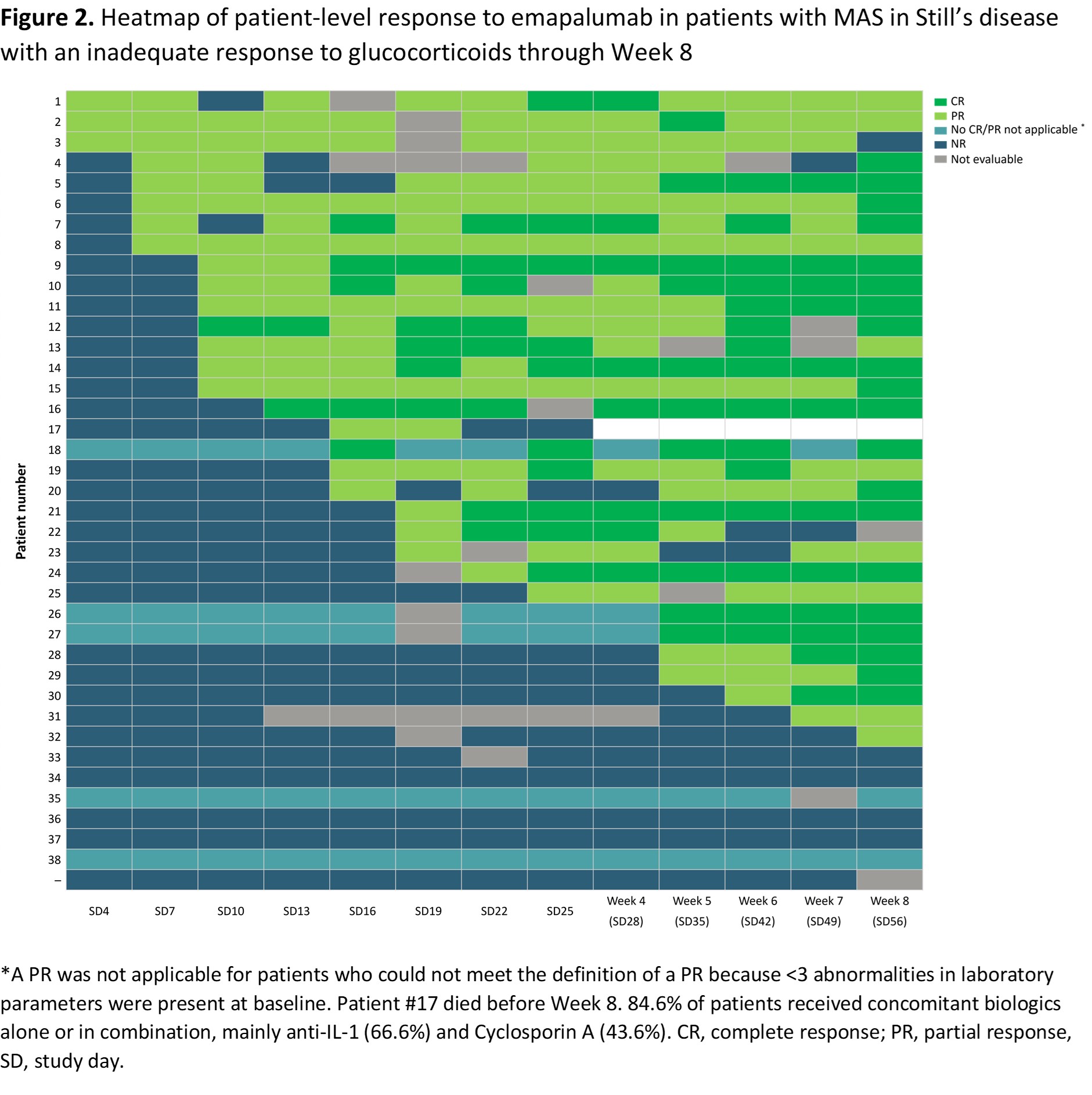 Figure 1. Time to first OR (n=38)*
Figure 1. Time to first OR (n=38)*
.jpg) Figure 2. Heatmap of patient-level response to emapalumab in patients with MAS in Still’s disease with an inadequate response to glucocorticoids through Week 8
Figure 2. Heatmap of patient-level response to emapalumab in patients with MAS in Still’s disease with an inadequate response to glucocorticoids through Week 8
To cite this abstract in AMA style:
Behrens E, Vastert S, anton J, Quartier P, Fautrel B, Brogan P, Elder M, Minoia F, Dolezalova P, Biesen R, Shimizu M, Ullmann U, Mahmood A, Danquah A, Burillo E, Petrimpol M, Mallett S, Jamieson B, GROM A, De Benedetti F. Baseline Pharmacodynamic Markers and Response to Emapalumab in Children and Adults with Macrophage Activation Syndrome (MAS) in Still’s Disease: Results from a Pooled Analysis of Two Prospective Trials [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/baseline-pharmacodynamic-markers-and-response-to-emapalumab-in-children-and-adults-with-macrophage-activation-syndrome-mas-in-stills-disease-results-from-a-pooled-analysis-of-two-prospecti/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/baseline-pharmacodynamic-markers-and-response-to-emapalumab-in-children-and-adults-with-macrophage-activation-syndrome-mas-in-stills-disease-results-from-a-pooled-analysis-of-two-prospecti/
