Session Information
Date: Saturday, November 16, 2024
Title: Muscle Biology, Myositis & Myopathies – Basic & Clinical Science Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
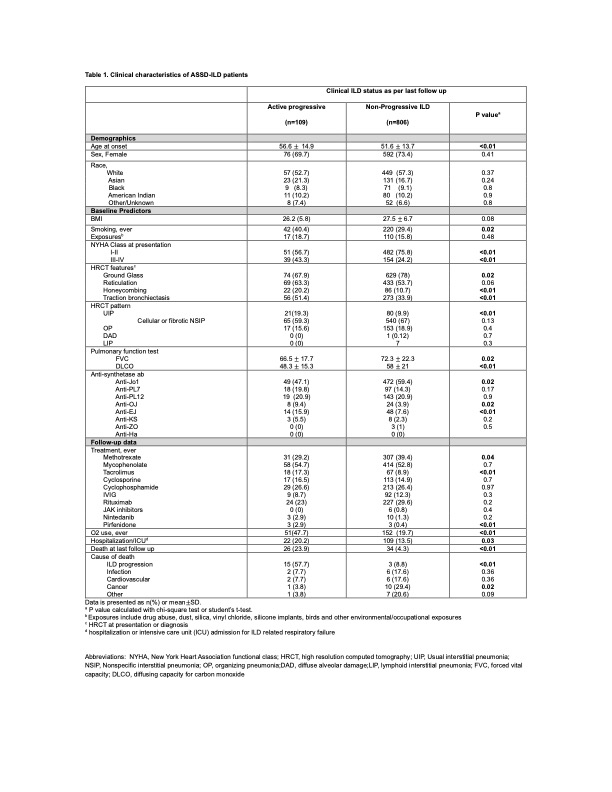
Background/Purpose: Anti-synthetase syndrome (ASSD) is a systemic autoimmune rheumatic disorder with significant heterogeneity. ILD is the most common cause of mortality and an important prognostic factor associated with poor survival. We aimed to describe the baseline ILD characteristics in patients with ASSD and clinical outcomes of ILD progression and death using the Classification of ASSD (CLASS) project database.
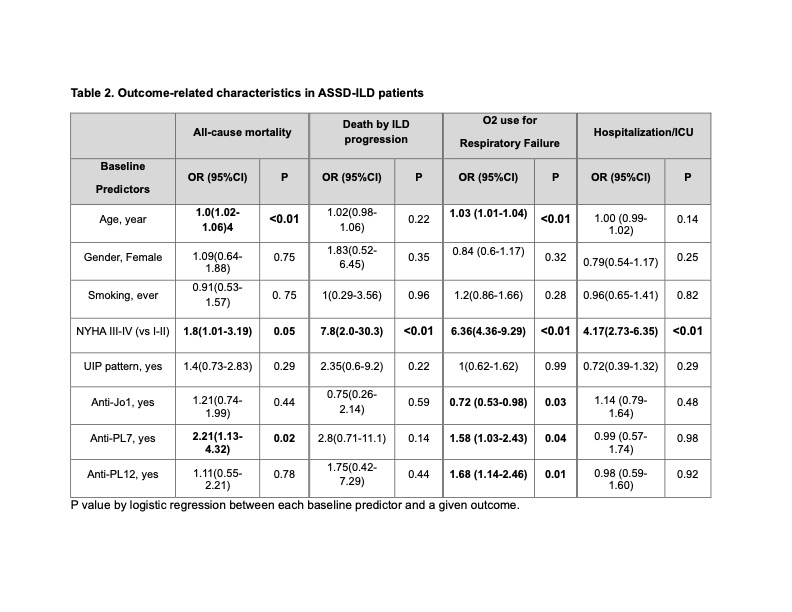
Methods: We utilized the CLASS database, with data entered by local physicians from 92 centers across 30 countries. The database includes comprehensive clinical, serological, radiographic, and physiological data. First, we compared baseline characteristics between ‘active progressive ILD’ or ‘non-progressive ILD’ (includes stable ILD and improved/resolved ILD) per local physician’s discretion at the last follow-up. Second, we performed association analysis using logistic regression between baseline predictor variables and the following outcomes: all-cause mortality, death by ILD progression, home O2 use for ILD, and hospitalization or intensive care unit (ICU) admission for ILD-related respiratory failure.
Results: 915 patients with ASSD-ILD who had follow-up data were included in the analysis (Table 1). Patients with active progressive ILD were older at ILD onset and, more frequently, smokers. The nonspecific interstitial pneumonia (NSIP) pattern was the most common HRCT ILD pattern in both groups. Patients with active progressive ILD had higher rates of fibrotic HRCT features (honeycombing, traction bronchiectasis) and a usual interstitial pneumonia (UIP) pattern, and baseline FVC and DLCO were significantly lower. The non-progressive ILD group had a higher proportion of anti-Jo1 antibodies. Follow-up data showed that patients with active progressive ILD had a significantly greater number of deaths, the majority due to ILD progression. The active progressive ILD group also had more respiratory failure and hospitalization or ICU admissions. Older age at ILD onset, NYHA class III/IV at baseline, and positive anti-PL7 and PL-12 antibodies were associated with a worse prognosis (Table 2).
Conclusion: Patients with active progressive ILD had older age at disease onset, were more frequently smokers, and had more fibrotic baseline HRCT features and lower FVC and DLCO at baseline. Age at ILD diagnosis, baseline NYHA class III-IV, and anti-PL7/PL-12 antibodies were associated with a worse prognosis. Early recognition of patients with poor prognostic factors may allow the implementation of more aggressive pharmacological intervention to improve mortality and other meaningful clinical outcomes.
To cite this abstract in AMA style:
Bozan F, Bae S, Rivero Gallegos D, Zanframundo G, Faghihi-Kashani S, Bauer Ventura I, Dourado E, Lim D, Loganathan A, sambataro G, Yoshida A, Bonella F, J Corte T, J Doyle T, fiorentino d, Gonzalez-Gay M, Hudson m, Kuwana M, Notarnicola A, Mammen A, McHugh N, Miller F, Montecucco C, Oddis C, Rojas-Serrano J, Schmidt J, Scire C, Villar A, Werth V, Aggarwal R, Cavagna L. Baseline Interstitial Lung Disease (ILD) Characteristics and Outcomes in Patients with Anti-synthetase Syndrome Related ILD (ASSD-ILD): Analysis from the “Classification Criteria for Anti-synthetase Syndrome (CLASS)” Project Database [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/baseline-interstitial-lung-disease-ild-characteristics-and-outcomes-in-patients-with-anti-synthetase-syndrome-related-ild-assd-ild-analysis-from-the-classification-criteria-for-anti-synt/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/baseline-interstitial-lung-disease-ild-characteristics-and-outcomes-in-patients-with-anti-synthetase-syndrome-related-ild-assd-ild-analysis-from-the-classification-criteria-for-anti-synt/