Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: Methotrexate (MTX) is the most commonly used drug in rheumatoid arthritis (RA). 30% of patients fail to respond to the drug or suffer from adverse events. Therefore, there is a need to identify determinants of MTX response and adverse events in RA. MTX is an antagonist for folate and interferes with folate homeostasis. We investigated whether folate-related biomarkers, including homocysteine (HCy), vitamin B6, B12 and folate, measured at baseline were associated with MTX response and adverse events over nine months of treatment.
Methods: The study included patients from two longitudinal cohorts who were diagnosed with RA according to the 2010 ACR criteria and were treated with MTX therapy: 285 patients from the treatment in Rotterdam Early Arthritis Cohort (tREACH) (1) and 99 from the Methotrexate in Rotterdam (MTX-R) study. Serum concentrations of HCy, B6, B12 and folate, and folate in erythrocytes were determined at baseline (t0). MTX response was assessed with the disease activity score (DAS)-28 at 0,3,6 and 9 months after MTX start and EULAR response criteria at 3,6 and 9 months. Adverse events were defined as gastro-intestinal intolerance and overall complaints at 3,6 and 9 months. Using DAS 28 as outcome measure, analysis of covariance (ANCOVA), adjusted for age, gender, DAS28 at baseline, MTX dose, MTX route of administration, other DMARDs, NSAIDs, steroids and study cohort, was used. EULAR and adverse events were analyzed with logistic regression. Biomarkers were analyzed as continuous variables, quintiles and tertiles.
Results: Concentrations at baseline (mean/SD) were: homocysteine: 12.9 μmol/l (5.9), B6: 93 nmol/l (97), B12: 322 pmol/l (131), folate in serum: 23 nmol/l (35) and folate in erythrocytes: 1007 nmol/l (450). DAS28 was 4.76 (1.26) at baseline and 3.08 (1.20), 2.91 (1.22), 2.68 (1.16) at 3,6 and 9 months. 52%,26% and 24% had gastro-intestinal complaints at 3, 6 and 9 months, respectively. 46%, 49% and 47% of the patients reported one or more adverse events at 3 6 and 9 months of follow-up.
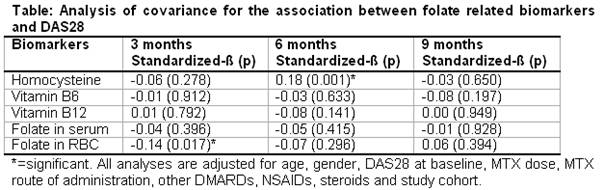
None of the biomarkers (defined as continuous measure) were associated with DAS28 at all 3 time-points (table). For HCy we only found a positive association at 6 months (standardized-ß = 0.18, p = 0.001), whereas folate in erythrocytes was only associated negatively with DAS28 at 3 months (st-ß = -0.14, p = 0.017). Analyses with biomarkers divided into quintiles or tertiles showed similar results. In addition, no associations were found between any of the baseline biomarkers and EULAR response criteria or adverse events.
Conclusion: In this first longitudinal study, folate-related biomarkers were unrelated to MTX treatment outcome in RA.
Funds: RDJ: Dutch Arthritis Association (nr. 06-02-402, 09-01-402).
Reference: 1. De Jong PH, et al. Ann Rheum Dis. 2012 Jun 7.
Disclosure:
M. C. F. J. De Rotte,
None;
S. M. F. Pluijm,
None;
M. Bulatovic,
None;
J. M. W. Hazes,
None;
R. De Jonge,
Dutch Arthritis Association,
2.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/baseline-folate-related-biomarkers-in-serum-and-erythrocytes-are-not-associated-with-methotrexate-response-and-adverse-events-in-rheumatoid-arthritis/