Session Information
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Systemic Lupus Erythematous (SLE) is a clinically and biologically diverse disease, for which only one new therapy has been approved in the past 60 years. In a phase 2 trial of mild-to-moderate SLE patients, ustekinumab (UST) improved clinical and laboratory measures of disease activity compared with placebo (PBO).1 We previously reported an association of IFN-g reduction with response to UST,2 suggesting an impact on the IL12/Th1 axis. To extend these findings, we performed unbiased transcriptomic analysis from baseline whole blood samples to identify genes that discriminate UST responders (UST-R) from non-responders (UST-NR) using the primary endpoint of Systemic Lupus Erythematosus Responder Index (SRI)-4 at week 24 to define response.
Methods: UST was studied in a Ph2 PBO-controlled study of 102 patients with seropositive SLE and active disease despite standard therapy. Patients were randomized 3:2 to receive IV UST 6 mg/kg or placebo followed by subcutaneous injections of UST 90mg or placebo every 8 weeks. Whole blood gene expression at baseline was measured via microarray using RNA samples from 100 patients, as samples from 2 patients failed quality control. An unbiased approach was used to identify gene signatures present at baseline that associate with UST response. Recombinant IL-12 or IL-23 was incubated in vitro with whole blood from 6 healthy donors for 24h and RNA-Seq was performed to determine the effect of these treatments on representative genes comprising the UST response signature.
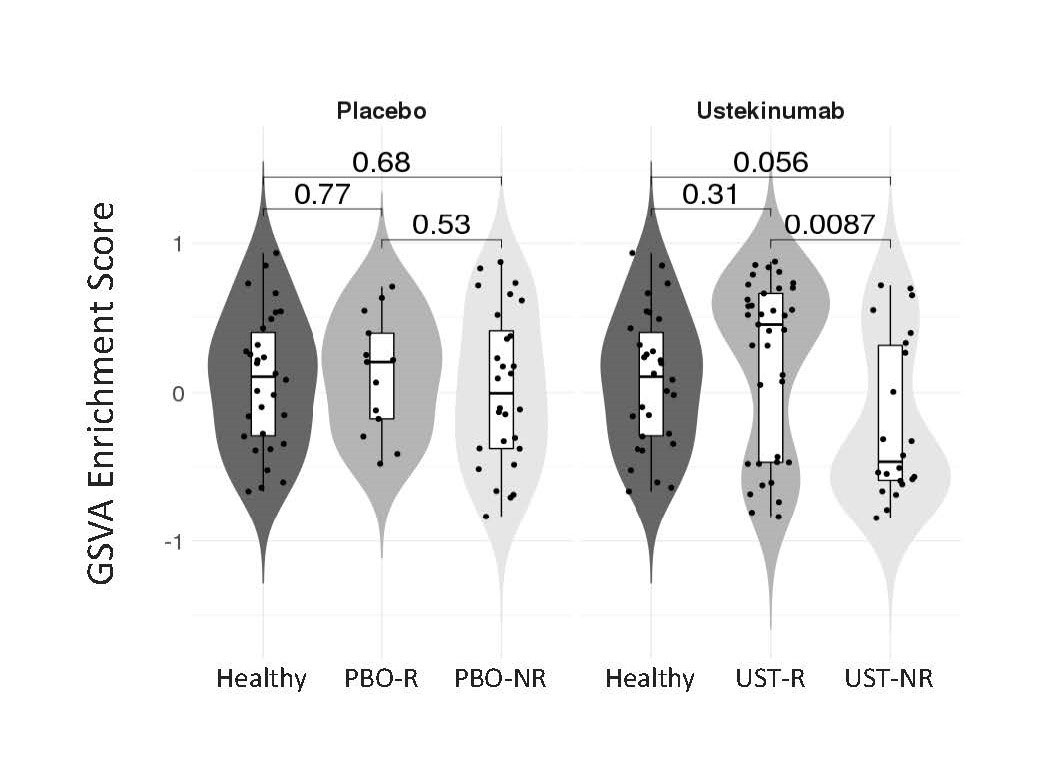
Results: A non-biased machine learning algorithm identified a 9-gene whole blood signature composed primarily of cytotoxic cell-associated transcripts (PRF1, KLRD1, GZMH, NKG7, GNLY, FGFBP2, TRGC2, TARP, TRGV2) that was enriched at baseline in UST-R vs UST-NR. By Gene Set Variation Analysis, the cytotoxic signature enrichment in UST-NR was less at baseline than both UST-R and a healthy control cohort (P=0.0087, P=0.056, respectively), whereas UST-R cytotoxic gene enrichment was similar to healthy controls (P=0.31). No significant difference in cytotoxic signature enrichment was observed at baseline between PBO responders and PBO non-responders or healthy controls (Figure). Enrichment levels of the cytotoxic gene signature remained stable over time in PBO and UST-NR groups while a trend of decreased cytotoxic signature was observed in UST-R, although never reaching levels seen in UST-NR. To begin to understand the relationship between IL-12 and IL-23, the targets of UST, and the cytotoxic signature, whole blood was stimulated with these cytokines in vitro. Recombinant IL-12, but not IL-23, resulted in increased expression of representative members of this cytotoxic gene signature.
Conclusion: We identified a novel cytotoxic signature in baseline blood samples that associated with UST response in SLE. The observation that IL-12 can increase this signature in vitro and that IL-12 is a robust inducer of cytotoxic cell activity3 as well as IFN-γ3 suggests an important role of IL-12 blockade in the mechanism of action of UST in SLE.
- van Vollenhoven RF. Lancet. 2018;392:1330-39
- Jordan. ACR 2018 Abstract # 2951
- G. Trinchieri. Nat Rev Immunol. 2003;3:133-46
To cite this abstract in AMA style:
Seridi L, Cesaroni M, Loza M, Schreiter J, Sweet K, Orlovsky Y, Baribaud I, Orillion A, Lipsky P, van Vollenhoven R, Hahn B, Tsokos G, Chevrier M, Rose S, Baribaud F, Jordan J. Baseline Cytotoxic Gene Expression Associates with Ustekinumab Response in Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/baseline-cytotoxic-gene-expression-associates-with-ustekinumab-response-in-systemic-lupus-erythematosus/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/baseline-cytotoxic-gene-expression-associates-with-ustekinumab-response-in-systemic-lupus-erythematosus/