Session Information
Date: Monday, November 13, 2023
Title: (1052–1081) Immunological Complications of Medical Therapy Poster
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Immune checkpoint inhibitors (ICI) can cause inflammatory arthritis (IA) of varying severity. Many patients with ICI-IA require immunosuppression beyond corticosteroids, but there is no way to identify these patients at initial presentation. We determined baseline risk factors for requiring immunosuppression and having persistent arthritis in ICI-IA.
Methods: participants were adults with rheumatologist diagnosed ICI-IA. The primary outcome was requirement of conventional synthetic (cs) or biologic (b) DMARDs; other outcomes were persistence of IA >6 months after ICI cessation and requirement of corticosteroids. Logistic regression models evaluated associations between clinical/genetic features and primary and secondary outcomes, with adjustment for potential confounders, as appropriate. Finally, we evaluated the relationship between maximum prior dose of steroids and primary and secondary outcomes with simple logistic regression.
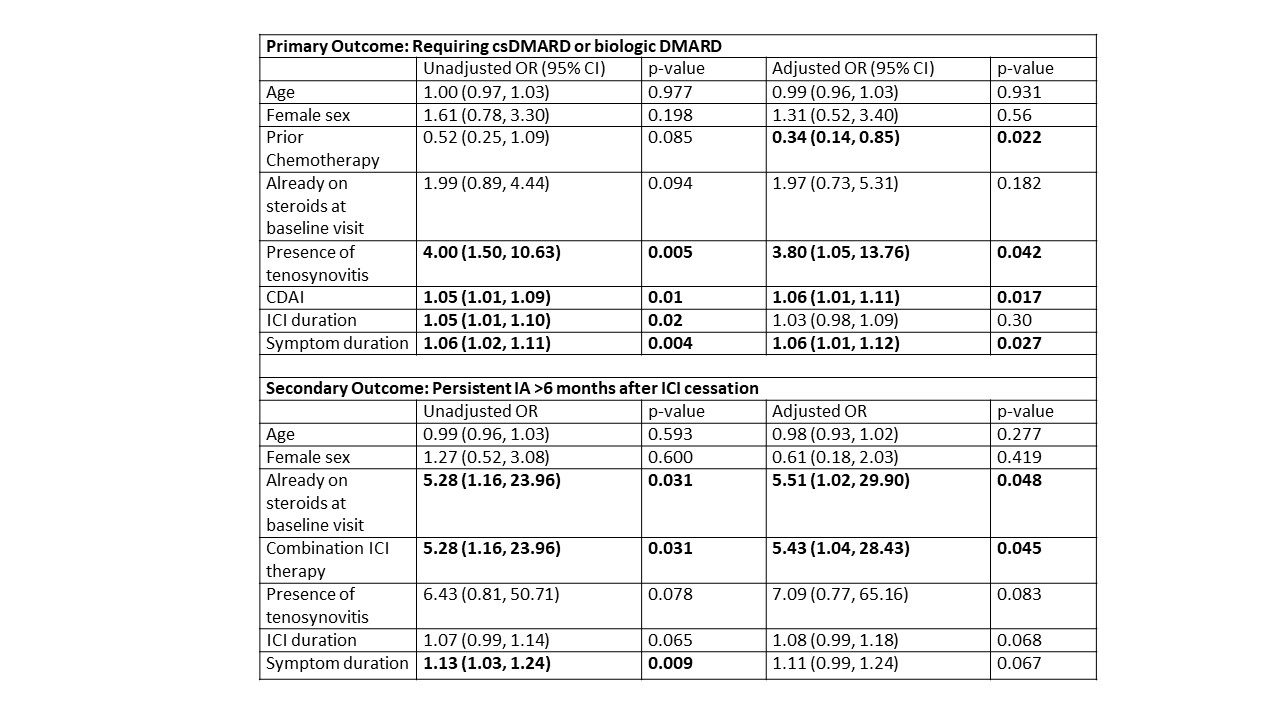
Results: 126 patients with ICI-IA were included; 53 patients (42%) required a csDMARD/bDMARD (demographic and clinical features by DMARD group in Table 1).In univariate logistic regressions, higher CDAI, tenosynovitis, longer symptom duration before first rheumatology visit, and longer ICI duration were significantly associated with a higher likelihood of requiring DMARDs; there was a trend toward those treated with prior chemotherapy being less likely to need DMARDs (Table 2). After adjustment, tenosynovitis, longer symptom duration, and higher CDAI remained associated with requiring DMARDs, while those with prior chemotherapy were significantly less likely to require DMARDs (Table 2). Combination anti-CTLA-4/PD-1 therapy and steroid use at baseline were associated with a higher risk of persistent IA (Table 2). For 98 patients with HLA typing data, there was no significant association with HLA DRB1 shared epitope alleles or HLA B27 and primary or secondary outcomes. For 62 patients reporting systemic steroid use after starting ICI therapy, but prior to their baseline visit with rheumatology, there were no differences between those treated with low dose (prednisone or equivalent ≤ 10 mg/daily), moderate dose (10 mg< prednisone daily ≤60 mg), or high dose (prednisone daily > 60 mg) corticosteroids in either requiring a csDMARD/biologic or having persistent ICI-IA.
Conclusion: Higher levels of disease activity, tenosynovitis, and longer symptom duration prior to baseline were associated with requiring DMARDs for ICI-IA, while those treated previously with chemotherapy were less likely to require DMARDs. Known genetic risk factors for traditional forms of IA were not predictive. The presence of risk factors for severe disease at baseline may indicate a role for higher initial steroid dose, earlier rheumatology referral, and adoption of immunosuppression beyond steroids to improve outcomes.
To cite this abstract in AMA style:
Cappelli L, Kamal o, Jones M, Bingham C, Shah A. Baseline Clinical Features, but Not Shared Epitope or HLA B27, Predict Severe Outcomes for Immune Checkpoint Inhibitor-induced Inflammatory Arthritis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/baseline-clinical-features-but-not-shared-epitope-or-hla-b27-predict-severe-outcomes-for-immune-checkpoint-inhibitor-induced-inflammatory-arthritis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/baseline-clinical-features-but-not-shared-epitope-or-hla-b27-predict-severe-outcomes-for-immune-checkpoint-inhibitor-induced-inflammatory-arthritis/