Session Information
Date: Wednesday, November 13, 2019
Title: 6W010: Pediatric Rheumatology – Clinical III: Juvenile SLE & Dermatomyositis (2864–2869)
Session Type: ACR Abstract Session
Session Time: 9:00AM-10:30AM
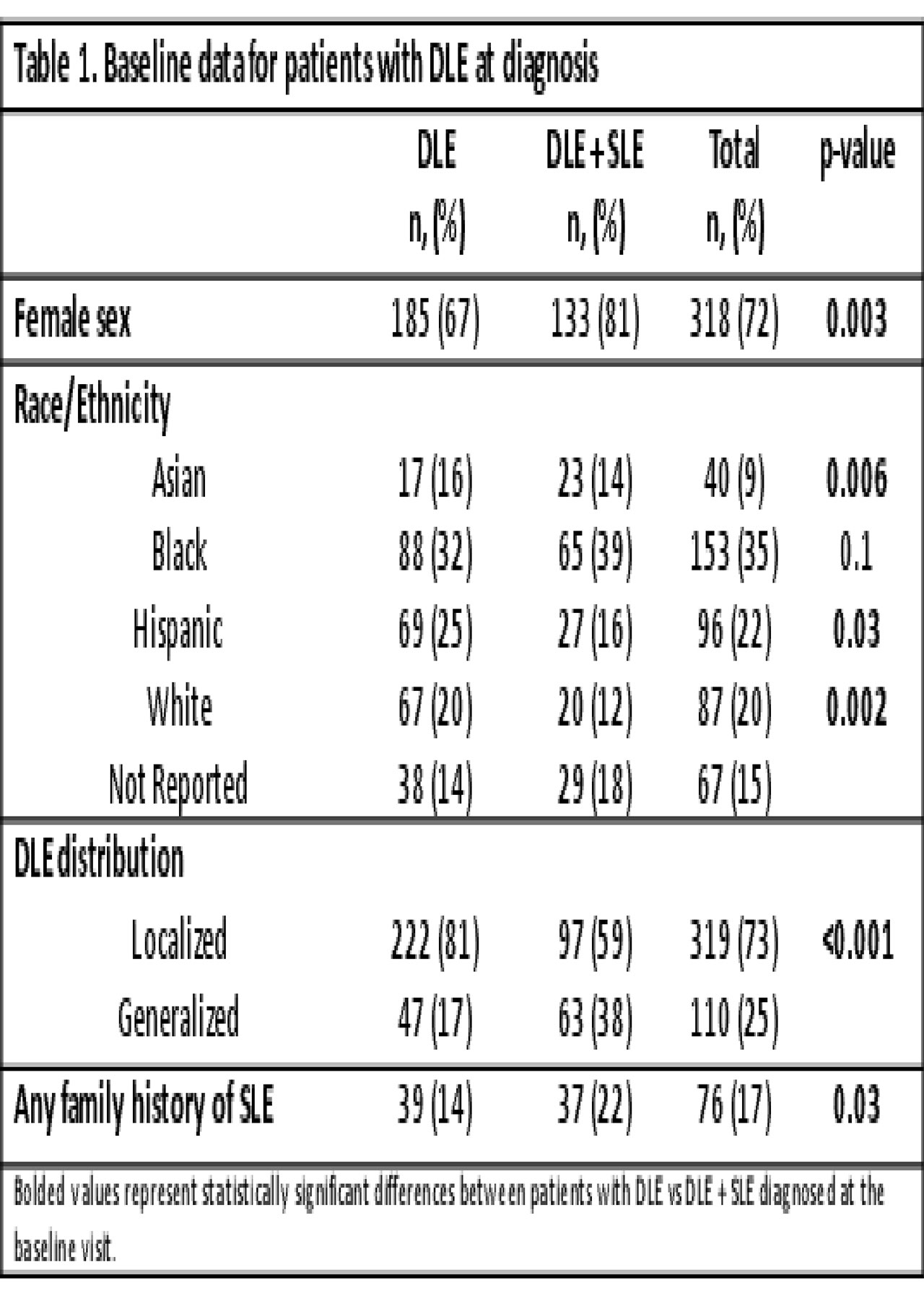
Background/Purpose: DLE is a rare, disfiguring disorder in children. Small retrospective studies suggest 20-25% of patients progress to SLE. Progression risk factors are poorly understood, but DLE has been associated with delay in SLE diagnosis and reduced access to care. This multicenter retrospective cohort study aimed to describe baseline characteristics and clinical phenotypes of pediatric DLE patients at diagnosis.
Methods: Medical records at eighteen sites were reviewed for pediatric dermatology and rheumatology patients with DLE. For inclusion, patients required clinical and/or histopathologic findings consistent with DLE. Baseline data were collected at the first documented visit including sociodemographic data, ACR/SLICC SLE criteria (i.e. DLE+SLE), date of DLE onset/diagnosis, DLE distribution, family history, comorbidities, and treatment. Outcome variables included ACR (primary outcome) /SLICC SLE criteria. Rates of progression from skin-limited DLE (DLE) to SLE (DLE+SLE) were evaluated. Analysis included descriptive statistics, chi-square and Wilcoxon tests.
Results: Out of >1,000 patients reviewed, 441 met inclusion criteria. The cohort was predominantly female (72%) and racially/ethnically diverse (Table 1). A minority presented at baseline with SLE based on ACR and SLICC criteria, respectively (n=165, 37%; n=183, 42%). DLE+SLE patients were older (median 13.7y vs 10.2y) with shorter time from DLE onset to diagnosis (median 2 mo vs 7 mo), compared to DLE patients (p< 0.001). DLE patients presented with low incidence of renal involvement, serositis, seizures or psychosis (p< 0.001, Table 2). DLE+SLE patients had more positive serologies and higher-titer ANAs (p< 0.001, Table 3), although 5% were ANA negative. Among 231 DLE patients with³1 follow up visit, median follow-up was 2.7 y (range 0-13.9y) with 747 total subject-years. Progression to SLE occurred in 20% and 25% of patients based on ACR and SLICC criteria, respectively.
Conclusion: To date, this is the largest investigation of pediatric DLE. Patients with DLE + SLE were most likely to present in adolescence with abnormal serologies and end-organ disease. Progression of DLE to SLE occurred at rates consistent with previous literature. All patients with DLE require SLE surveillance at diagnosis and regular follow-up, particularly during adolescence. Limitations include the retrospective study design with potential for misclassification, and analysis restricted to the baseline visit. Further analysis of follow up visits will evaluate for baseline risk factors and biomarkers of evolving SLE, as well as timing of progression, identifying DLE patients at highest risk for systemic disease.
To cite this abstract in AMA style:
Ezeh N, Buhr K, Nguyen C, Al Ahmed O, Ardoin S, Barton V, Bell S, Brandling-Bennett H, Castelo-Soccio L, Chiu Y, Chong B, Co D, Lara-Corrales I, Cintosun A, Diaz L, Elman S, Fernandez Faith E, Garcia-Romero M, Grossman-Kranseler J, Hersh A, Hogeling M, Hudson A, Hunt R, Ibler E, Marques M, Monir R, Oza V, Paller A, Putterman E, Rodriguez-Salgado P, Schoch J, Truong A, Wang J, Wine Lee L, Vleugels R, Klein-Gitelman M, von-Scheven E, Werth V, Ardalan K, Arkin L. Baseline Clinical and Serological Findings in Pediatric-Onset Discoid Lupus Erythematosus: Analysis of a Multicenter Retrospective Cohort Study [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/baseline-clinical-and-serological-findings-in-pediatric-onset-discoid-lupus-erythematosus-analysis-of-a-multicenter-retrospective-cohort-study/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/baseline-clinical-and-serological-findings-in-pediatric-onset-discoid-lupus-erythematosus-analysis-of-a-multicenter-retrospective-cohort-study/