Session Information
Date: Monday, October 22, 2018
Title: Rheumatoid Arthritis – Diagnosis, Manifestations, and Outcomes Poster II: Diagnosis and Prognosis
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Tofacitinib is an oral Janus kinase inhibitor for the treatment of RA. ORAL Strategy (NCT02187055) was a Phase 3b/4, 1-year, double-blind, triple-dummy, active comparator-controlled trial in patients (pts) with moderate to severe RA who were inadequate responders (IR) to MTX.1 In ORAL Strategy, tofacitinib + MTX showed non-inferiority to adalimumab + MTX, clinically important improvement was seen with tofacitinib monotherapy, although non-inferiority vs tofacitinib + MTX or adalimumab + MTX was not demonstrated. The current investigation was a post hoc analysis of baseline (BL) characteristics in patients from the tofacitinib 5 mg BID monotherapy group in ORAL Strategy.
Methods: The (binary) efficacy outcome measure was achievement of low disease activity (LDA) at Month (M)6. LDA was defined using three different response variables separately: Simplified Disease Activity Index (SDAI)≤11, Clinical DAI (CDAI)≤10, or Disease Activity Score in 28 joints, erythrocyte sedimentation rate (DAS28-4[ESR]) <3.2.2 BL characteristics by response/no response at M6 were summarized; BL covariates were evaluated for association with each efficacy outcome using univariate logistic regression analysis without adjustment for other covariates. Category cut-offs of BL variables were defined by existing publications, clinical practice, or expert opinion provided to the study sponsor.
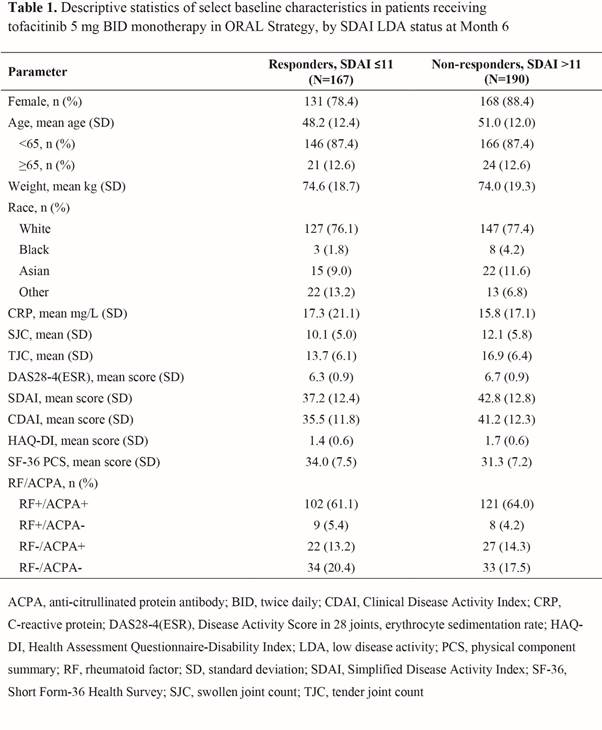
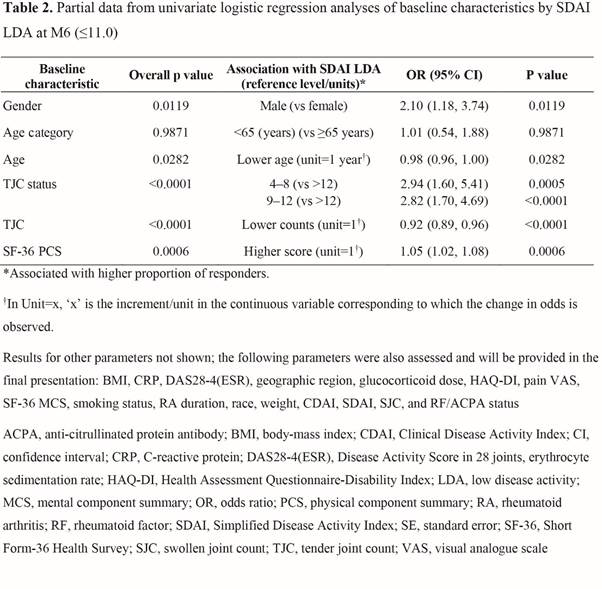
Results: Overall, 357 pts were included in the analysis for SDAI; Table 1 shows selected BL characteristics by SDAI LDA status at M6. From univariate analysis, more BL covariates showed significant association with M6 SDAI LDA (Table 2) than CDAI or DAS28-4(ESR) LDA. M6 CDAI LDA univariate results showed similar trends to SDAI LDA, but with fewer factors showing associations. The BL covariates significantly associated with any of the three LDA measures at M6 included age, gender, disease severity (eg, tender joint count [TJC], DAS28-4[ESR], SDAI, and rheumatoid factor/anti-citrullinated protein antibody status) and Short Form-36 Health Survey physical component summary (SF-36 PCS; Table 2).
Conclusion: This post hoc analysis suggests that for MTX-IR pts who use tofacitinib monotherapy, age, gender, and BL disease severity (e.g. by TJC) and SF-36 PCS score may be associated with achieving LDA at M6. Multivariable analysis will be performed to further explore the associations; additional prospective research is warranted to confirm the findings.
1. Fleischmann R et al. Lancet 2017; 390: 457-68.
2. Singh JA et al. Arthritis Care Res 2016; 68: 1-25.
To cite this abstract in AMA style:
Kaine J, Kivitz AJ, Mysler E, Iikuni N, Fan H, Diehl A, Paulissen J, Murray CW. Baseline Characteristics of Methotrexate Inadequate Responder Patients with RA Who Achieved Low Disease Activity with Tofacitinib Monotherapy [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/baseline-characteristics-of-methotrexate-inadequate-responder-patients-with-ra-who-achieved-low-disease-activity-with-tofacitinib-monotherapy/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/baseline-characteristics-of-methotrexate-inadequate-responder-patients-with-ra-who-achieved-low-disease-activity-with-tofacitinib-monotherapy/