Session Information
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: The Phase IIIb Assessing Very Early Rheumatoid arthritis Treatment (AVERT)-2 trial (NCT02504268) is evaluating SC abatacept (ABA) + MTX versus ABA placebo (PBO) + MTX in adults with early RA.1 AVERT-2 was designed to demonstrate that ABA + MTX could achieve higher rates of SDAI remission versus ABA PBO + MTX and to test a clinically meaningful withdrawal strategy. Identifying factors associated with response may help guide treatment decisions, including initial treatment choice and de-escalation of therapy. We investigated baseline characteristics that were associated with sustained SDAI remission (≤3.3 at Weeks 40 and 52), therefore allowing patients to enter the de-escalation period, in AVERT-2.
Methods: For the induction period (IP), patients were randomized 3:2 to SC ABA (125 mg weekly) + MTX or ABA PBO + MTX for 56 weeks. Following the IP, patients entered a 48-week de-escalation period. Key inclusion criteria: age ≥18 years; RA diagnosis ≤6 months (ACR/EULAR 2010 criteria); ACPA+; CRP >3 mg/L or ESR ≥28 mm/h; TJC ≥3 and SJC ≥3; DMARD naive. Logistic models were used to explore potential factors associated with sustained SDAI remission by treatment arm in the cohort 1 analysis population (all randomized patients treated in the IP). Continuous variables were dichotomized based on median values before introduction to the models. A total of 30 baseline characteristics (demographics, disease/clinical endpoints, co-medication use, serum markers, patient-reported outcomes) were tested individually by logistic regression in a univariate analysis, and variables with p< 0.2 were entered into a multivariate model.
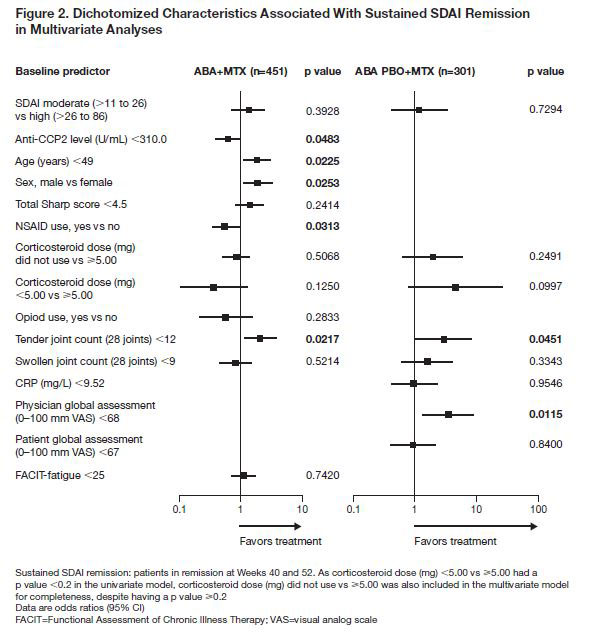
Results: Overall, 752 patients were randomized and treated in cohort 1 during the IP: 451 with ABA + MTX and 301 with ABA PBO + MTX. Baseline characteristics were similar across treatment arms. A total of 11 and 8 baseline characteristics, respectively, were identified as independently associated with sustained remission with ABA + MTX and ABA PBO + MTX (Fig. 1). Baseline characteristics associated with sustained remission by multivariate analysis (Fig. 2) with ABA + MTX were anti-CCP ≥310 U/mL, age < 49 years, male sex, NSAID naïve and TJC < 12. Baseline characteristics associated with sustained remission by multivariate analysis with ABA PBO + MTX were physician global assessment (PGA) < 68 and TJC < 12 (Fig. 2).
Conclusion: In this population of ACPA+ patients with early RA, several baseline characteristics were found to be associated with sustained SDAI remission in patients treated with abatacept + MTX: anti-CCP2 levels ≥310 U/mL, age < 49 years, TJC < 12, being NSAID naïve and/or being male. In patients treated with abatacept PBO + MTX, having PGA < 68 and/or TJC < 12 were associated with sustained SDAI remission. The identification of baseline characteristics associated with treatment response in patients with very early RA may provide additional insight into which patients may be suitable for de-escalation of therapy.
Reference:
- Emery P, et al. ACR Annual Meeting 2018: Poster 563.
Professional medical writing: Lola Parfitt, Caudex, funded by Bristol-Myers Squibb.
To cite this abstract in AMA style:
Emery P, Tanaka Y, Bykerk V, Bingham C, Huizinga T, Citera G, Huang K, Connolly S, Elbez Y, Lozenski K, Fleischmann R. Baseline Characteristics Associated with Sustained SDAI Remission Following Treatment with Abatacept in Combination with MTX Compared with Abatacept Placebo in Combination with MTX in ACPA Positive Patients with Early RA [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/baseline-characteristics-associated-with-sustained-sdai-remission-following-treatment-with-abatacept-in-combination-with-mtx-compared-with-abatacept-placebo-in-combination-with-mtx-in-acpa-positive-pa/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/baseline-characteristics-associated-with-sustained-sdai-remission-following-treatment-with-abatacept-in-combination-with-mtx-compared-with-abatacept-placebo-in-combination-with-mtx-in-acpa-positive-pa/