Session Information
Date: Monday, October 22, 2018
Title: Rheumatoid Arthritis – Treatments Poster II: PROs, Safety and Comorbidity
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Tofacitinib is an oral Janus kinase inhibitor for the treatment of RA. The purpose of this study is to describe the profile of patients (pts) with RA and clinically significant anemia, and the impact of tofacitinib on those with anemia.
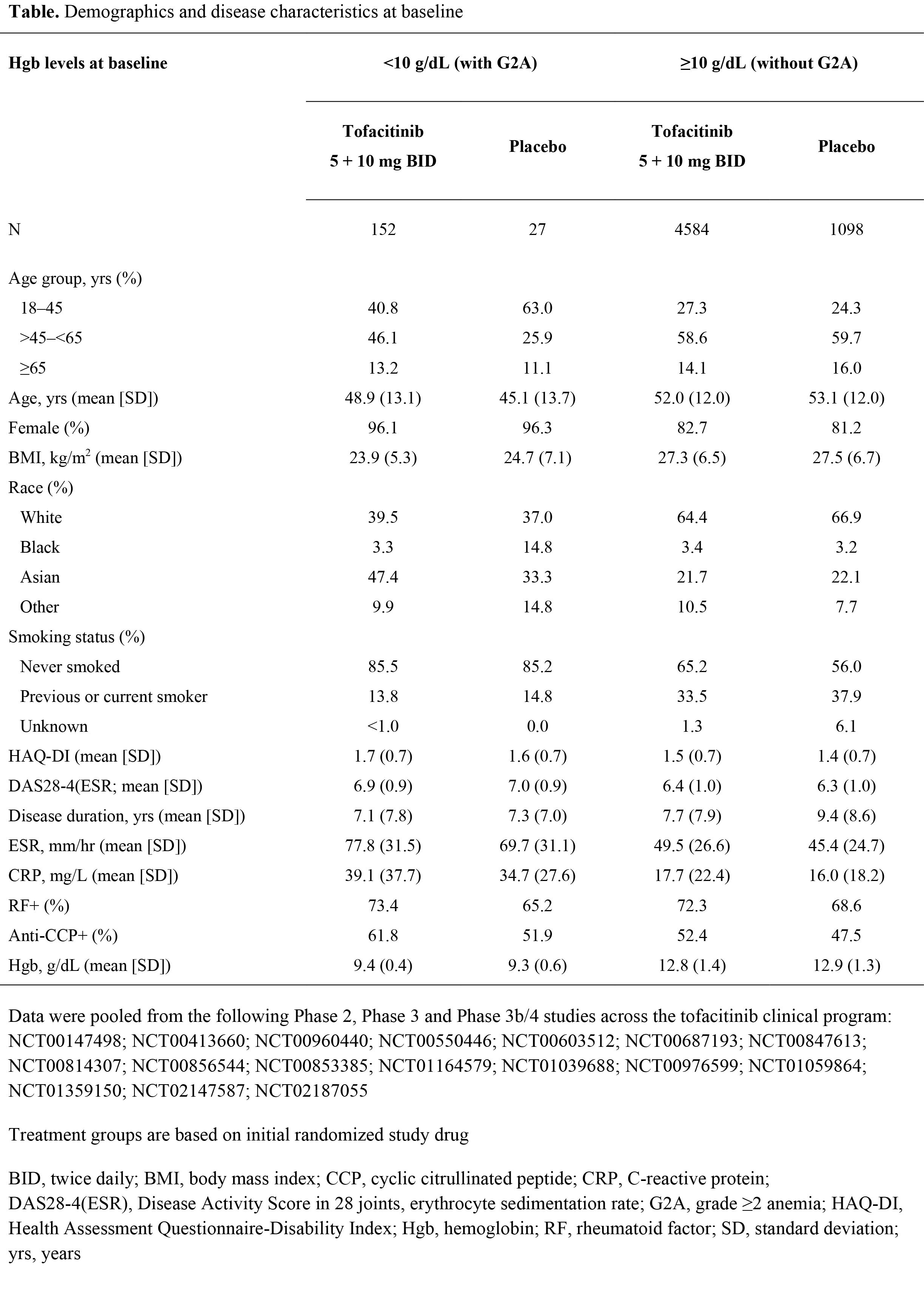
Methods: In this post hoc analysis, data were pooled from Phase (P)2, P3, and P3b/4 studies across the tofacitinib clinical program. Pts received tofacitinib 5 or 10 mg BID with/without background csDMARDs, or placebo (PBO). Pts with grade ≥2 anemia (G2A; Hgb <10 g/dL) at baseline (BL) were compared to pts without G2A (Hgb ≥10 g/dL) at BL. Demographic and BL characteristics, Hgb levels and efficacy (DAS28-4[ESR]) at Month (M)6, and treatment-emergent adverse events (TEAEs) were summarized descriptively.
Results: The proportion of pts with G2A at BL was similar for tofacitinib (3.2%, 152 of 4736 pts) and PBO (2.4%, 27 of 1125 pts) groups. Presence of G2A at BL was higher in those with female gender, Asian ethnicity, never smoker status, lower age and BMI, and higher CRP and ESR, compared to pts without G2A; RA duration was generally similar across groups (Table). Tofacitinib seemed to improve anemia more rapidly than PBO: among pts with G2A at BL, a lower proportion of those receiving tofacitinib had G2A at M1 and M3 compared with those receiving PBO (48.8 vs 75.0% and 36.1 vs 57.1%), while the proportions were similar at M6 (28.9 vs 30.6%). Among pts receiving tofacitinib, mean Hgb levels gradually increased from BL to M6 in those with G2A at BL (1.25 g/dL change), but not in those without G2A at BL (0.15 g/dL change). In pts receiving tofacitinib with and without G2A at BL, DAS28-4(ESR) scores decreased from BL to M6 by -2.40 and ‑2.42, respectively. DAS28-4(ESR) low disease activity rate at M6 was lower in tofacitinib-treated pts with G2A at BL than in those without G2A at BL (18.3 vs 28.4%). Among pts receiving tofacitinib, those with BL G2A had a higher incidence of TEAEs than those without BL G2A in the following MedDRA system organ classes (with incidence >20% in pts with and without BL G2A): gastrointestinal disorders (30.9% vs 22.5%) and infections and infestations (44.1% vs 39.0%).
Conclusion: In these studies, female gender, Asian ethnicity, never smoker status, low BMI and elevated ESR/CRP seemed to be associated with G2A at BL. G2A resolved within 6 months in most pts with RA receiving tofacitinib, while inflammation and disease activity (assessed by DAS28-4[ESR] scores) decreased. G2A appeared to resolve more rapidly in pts receiving tofacitinib than in those receiving PBO. These data suggest that tofacitinib can be a treatment option for pts with active RA and anemia. In pts with Hgb <8 g/dL or a decrease in Hgb >2 g/dL, however, it is recommended that tofacitinib dosing is interrupted until Hgb levels have normalized.1
1 US Food and Drug Administration. XELJANZ® (tofacitinib) highlights of prescribing information. Available at: http://labeling.pfizer.com/ShowLabeling.aspx?id=959. Accessed June 4, 2018.
To cite this abstract in AMA style:
Moeller B, Finckh A, Alvaro-Gracia JM, Scholz G, Aletaha D, Biondo F, Strengholt S, Rivas JL, Connell CA, Shi H. Baseline Characteristics and Outcomes in Patients with Anemia in Clinical Studies of Tofacitinib [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/baseline-characteristics-and-outcomes-in-patients-with-anemia-in-clinical-studies-of-tofacitinib/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/baseline-characteristics-and-outcomes-in-patients-with-anemia-in-clinical-studies-of-tofacitinib/