Session Information
Date: Sunday, November 8, 2020
Title: Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster III
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Tumor Necrosis Factor inhibitors (TNFi) have proven to be safe and effective in the treatment of axial spondyloarthritis (axSpA). However, they carry some disadvantages, such as adverse effects, patient burden, and costs, which could be reduced by treat-to-target (T2T) tapering. Although there is lack of high level evidence, guidelines suggest – based on experience in Rheumatoid Arthritis – that T2T tapering and discontinuation might be contemplated. However, there are no studies comparing T2T tapering and continuation in axSpA, with at least two studies ongoing (BIODOPT and DRESS-PS). As T2T treatment and tapering (BASDAI (Bath Ankylosing Spondylitis Disease Activity Index) guided) has been usual care in our axSpA population since 2012, this creates the opportunity to perform a retrospective controlled study.
Our objective was to assess the effect of T2T TNFi tapering on disease activity and TNFi dosage in axSpA patients with low disease activity (LDA).
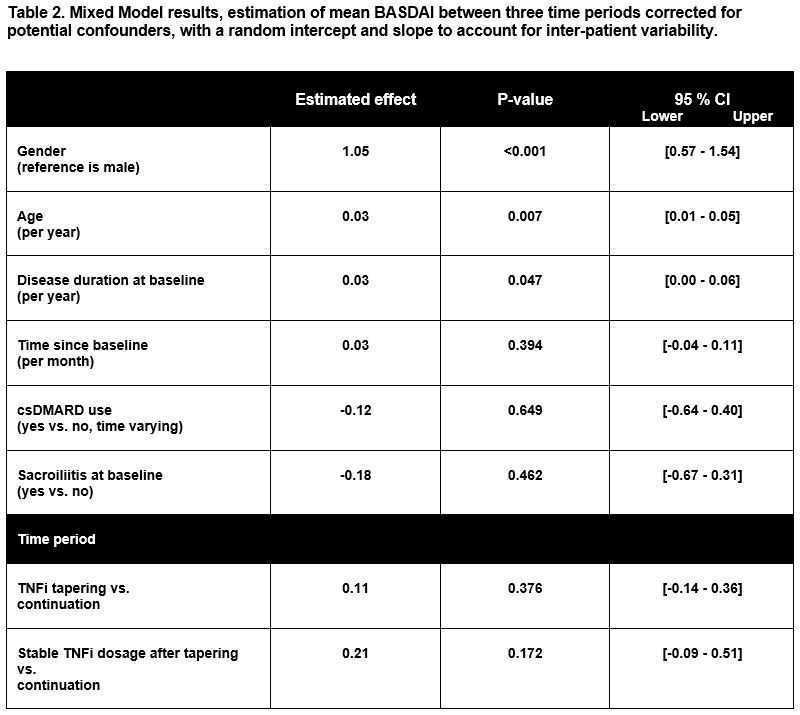
Methods: We performed a retrospective cohort study in all axSpA patients (clinical diagnosis) using TNFi who visited our outpatient clinic between April 2012 and October 2018. Patients with concomitant inflammatory disease preventing tapering were excluded and patients had to be eligible for tapering, defined as ≥ 6 months of TNFi treatment and ≥ 6 months of LDA (BASDAI < 4 or by judgement of physician and patient). Three different time periods were defined: i) continuation of TNFi; ii) TNFi tapering; iii) stable TNFi dosage after tapering. A mixed-model analysis was used to estimate mean BASDAI during these three time periods. This model included: age, gender, sacroiliitis, disease duration, and the following time-varying components: current dose reduction status (three time periods mentioned above), time since eligibility for tapering and use of concomitant conventional synthetic Disease-Modifying Antirheumatic Drugs (csDMARDs), and a random intercept and slope to account for inter-patient variability. Furthermore, a mean percentage of the Daily Defined Dose (%DDD) was calculated for each time period as secondary outcome.
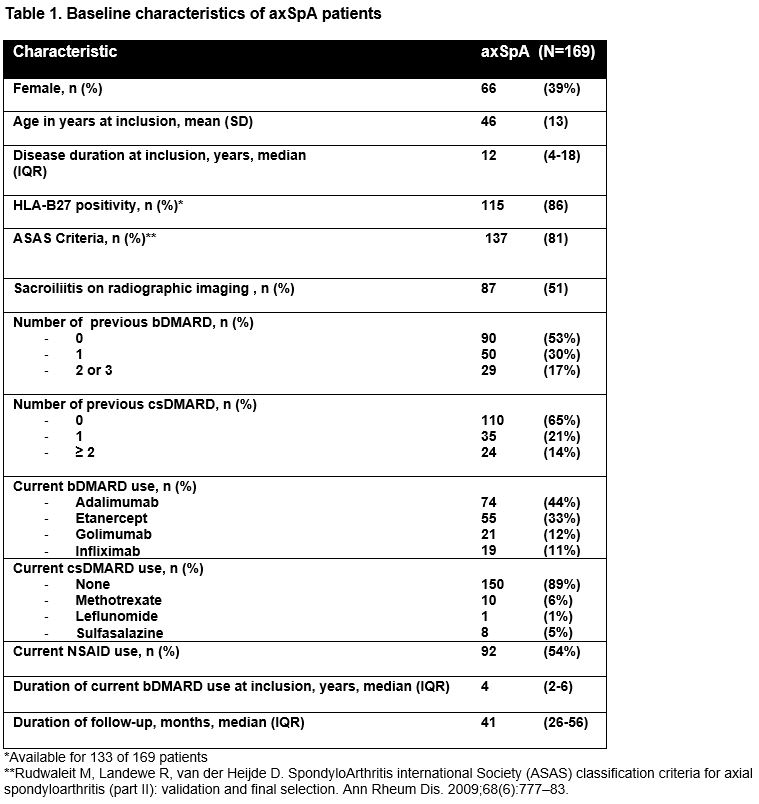
Results: 169 patients were included, with a mean of 6.4 BASDAI measurements, of whom 118 attempted dose reduction at least once during follow-up. Median follow-up duration was 31 months (Inter Quartile Range (IQR) 20-47) for patients who never attempted dose reduction and 45 months (IQR 31-56) for those who did (table 1). The mixed model showed no significant difference in BASDAI between the three time periods. Higher age, female gender and longer disease duration were significantly associated with a higher BASDAI score (table 2). The mean percentage of the daily defined dose for the three time periods was 96% for the continuation period; 59% in the TNFi tapering period and 69% in the stable TNFi dosage period.
Conclusion: T2T tapering of TNFi appears to have no detrimental effect on disease activity in axSpA patients, compared with full dose continuation, and results in reduction in drug exposure. Trials are needed to investigate tapering in a prospective and randomized manner.
To cite this abstract in AMA style:
den Broeder N, Mulder M, Wenink M, den Broeder A, Verhoef L, van den Hoogen F, Michielsens C. BASDAI Guided Treat-to-target Tapering of Tumor Necrosis Factor Inhibitors in Axial Spondyloarthritis: Results of the Retrospective TAPAS Study [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/basdai-guided-treat-to-target-tapering-of-tumor-necrosis-factor-inhibitors-in-axial-spondyloarthritis-results-of-the-retrospective-tapas-study/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/basdai-guided-treat-to-target-tapering-of-tumor-necrosis-factor-inhibitors-in-axial-spondyloarthritis-results-of-the-retrospective-tapas-study/