Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: The EULAR guidelines for Rheumatoid Arthritis (RA) patients advise to use a strategy aiming at a predefined target of disease activity (T2T). If this target is not achieved with csDMARD, adding a TNF-inhibitor (TNFi) or a JAK-inhibitor (JAKi) are advised options in cases with poor prognostic factors, obviously considering contraindications. While randomized clinical trials have provided insight into the relative efficacy and safety of TNFi and JAKi under trial conditions, the generalizability to real-life clinical practice remains unclear.
We aim to demonstrate the non-inferiority (NI) and, in case NI could be shown, subsequent superiority of a T2T strategy using baricitinib versus TNFi, after failure of csDMARDs, in a real-life setting.
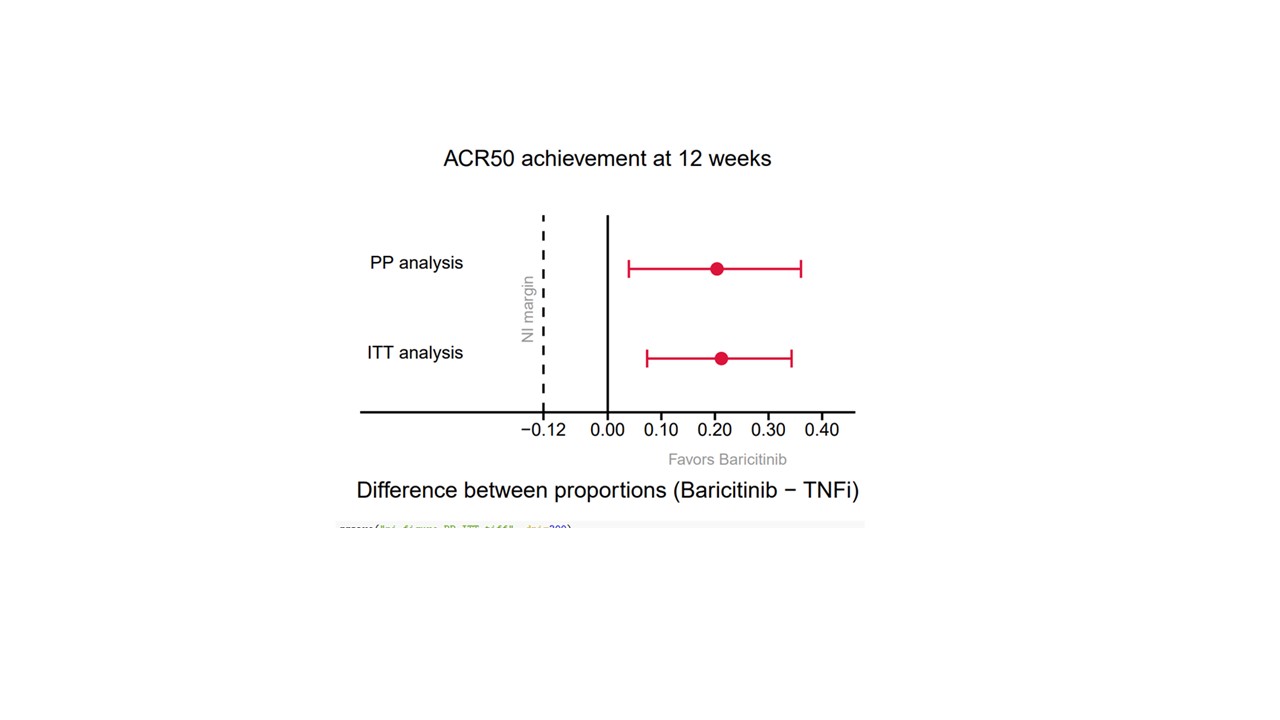
Methods: Biologic or targeted synthetic DMARD(b/tsDMARD)-naïve RA patients failing to respond to csDMARDs were eligible if they were pretreated according to T2T principles, had a disease duration ≤5 years and no contraindications to b/tsDMARD. All included patients were treated open label, according the T2T principle, at the discretion of their attending physician with either a TNFi (any type) or baricitinib. Patients were seen at baseline and 12-weekly until final follow-up (48 weeks). Full clinical assessment was performed at each visit and patients completed several PROMs. The primary endpoint was defined as NI of baricitinib versus TNFi with respect to the number of patients achieving ACR50 response at week 12. Subsequently, superiority testing was foreseen in case non-inferiority was shown. For the primary efficacy analysis, the ACR50 responses were compared, using 95% confidence intervals calculated using the Wilson score method for the difference in proportions. The non-inferiority margin for baricitinib was set at -12%. Linear mixed models were used to analyze continuous secondary outcomes over the study period of 48 weeks.
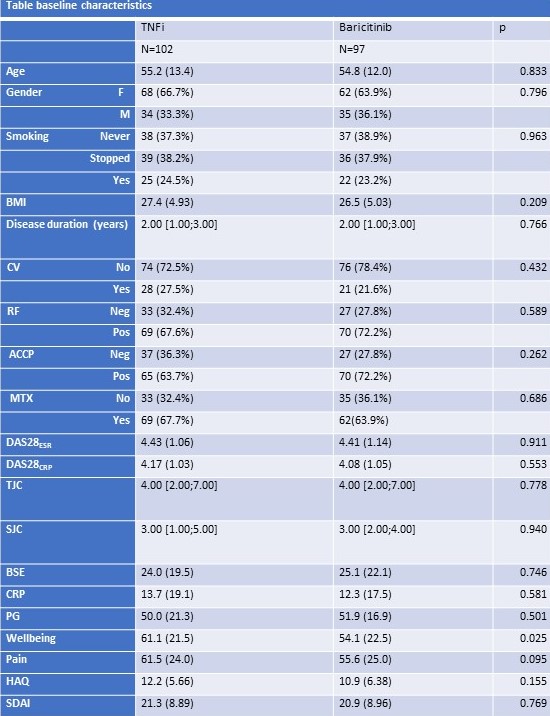
Results: 199 patients who received a first dose of either TNFi (n=102) or baricitinib (n=97) were included. Baseline characteristics were comparable between both groups (see table). At 12 weeks, the lower bound of the 95% confidence interval for the difference in the ACR50 response was above zero, in both the per-protocol and intention-to-treat analysis (figure 1). Hence, baricitinib was found to be non-inferior and statistically superior to TNFi in the analysis of the primary endpoint. Moreover, DAS28 remission (DAS28-CRP < 0.6) was achieved in 74% of baricitinib patients compared with 47% of TNFi patients (p< 0.001) at 12 weeks. All secondary clinical and PRO -measures over the study period of 48 weeks showed better responses, most often statistically superior, of Baricitinib over TNFi (figure 2 for DAS28-CRP).
Conclusion: Baricitinib was found to be non-inferior as well as superior to TNFi in terms of ACR50 response at 12 weeks in real-world csDMARD refractory RA patients. Analysis of the secondary endpoints on disease activity and PROMs, were consistently in favor of the group that started Baricitinib.
To cite this abstract in AMA style:
Van de Laar M, Oude Voshaar M, ten Klooster P, Tedjo D, van de laar c. Baricitinib versus TNF-inhibitors in Patients with Active Rheumatoid Arthritis After Failure of CsDMARDs: A Pragmatic, Multicenter, Real-Life Study in a Treat-to-Target Setting [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/baricitinib-versus-tnf-inhibitors-in-patients-with-active-rheumatoid-arthritis-after-failure-of-csdmards-a-pragmatic-multicenter-real-life-study-in-a-treat-to-target-setting/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/baricitinib-versus-tnf-inhibitors-in-patients-with-active-rheumatoid-arthritis-after-failure-of-csdmards-a-pragmatic-multicenter-real-life-study-in-a-treat-to-target-setting/