Session Information
Date: Sunday, November 8, 2020
Title: RA – Treatments Poster III: PROs, Biomarkers, Systemic Inflammation & Radiographs
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Baricitinib (BARI) improved patient-reported outcomes (PROs) in patients with insufficient response or intolerance to ≥1 tumor necrosis factor inhibitors (TNFi) or other biological disease-modifying antirheumatic drugs (bDMARDs) as well as in patients with inadequate response (IR) to conventional synthetic DMARDs (csDMARDs).1,2 The purpose of this analysis was to determine the association between improvement in PROs (pain, physical function, fatigue, and morning joint stiffness) and disease activity (measured using Clinical Disease Activity Index [CDAI] at 12 weeks. We evaluated whether BARI 2-mg provided greater PRO improvement compared to placebo (PBO), across all levels of disease activity.
Methods: Data for these post-hoc analyses were taken from two phase 3 studies, RA-BEACON (NCT01721044; bDMARD-IR patients) and RA-BUILD (NCT01721057; csDMARD-IR patients). Pain was assessed on a 0-100 mm visual analog scale; physical function was assessed using the Health Assessment Questionnaire-Disability Index (HAQ-DI); and fatigue was measured using the Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F) scale. Duration of morning joint stiffness (MJS, minutes) was also reported by the patient. Disease activity was assessed with the Clinical Disease Activity Index (CDAI) and categorized as: remission (≤2.8), low disease activity (LDA; >2.8 to ≤10), moderate disease activity (MDA; >10 to ≤22), high disease activity (HDA; >22). To evaluate the extent of benefit in PROs with BARI across disease activity levels at Week 12 relative to PBO, we used linear regression to model the relationship between change in PROs at Week 12 (response), and CDAI values at Week 12 (primary explanatory variable). Two additional variables included were treatment group and the interaction term between treatment and CDAI. We used last observation carried forward to impute missing values.
Results: At 12 weeks in bDMARD-IR patients, BARI 2-mg demonstrated greater improvements in pain, physical function, fatigue and MJS duration across all disease activity levels vs. placebo group (Figure 1). In csDMARD-IR patients, BARI 2-mg provided greater improvement in PROs (pain, HAQ-DI, MJS and fatigue) for patients who achieved disease control (remission or LDA) compared to placebo group. Greater reduction in pain and MJS duration were also observed in BARI 2-mg patients with MDA or HDA relative to placebo (Figure 2).
Conclusion: BARI 2-mg provided greater improvements in pain, physical function, fatigue and MJS duration across all levels of disease activity in bDMARD-IR patients and csDMARD-IR patients who achieved remission or LDA. Greater reduction in pain and MJS duration were also observed in csDMARD-IR patients with MDA or HDA at Week 12.
References:
- Smolen et al. Ann Rheum Dis. 2017;76(4):694-700.
- Emery et al. RMD Open. 2017;3(1):e000410.
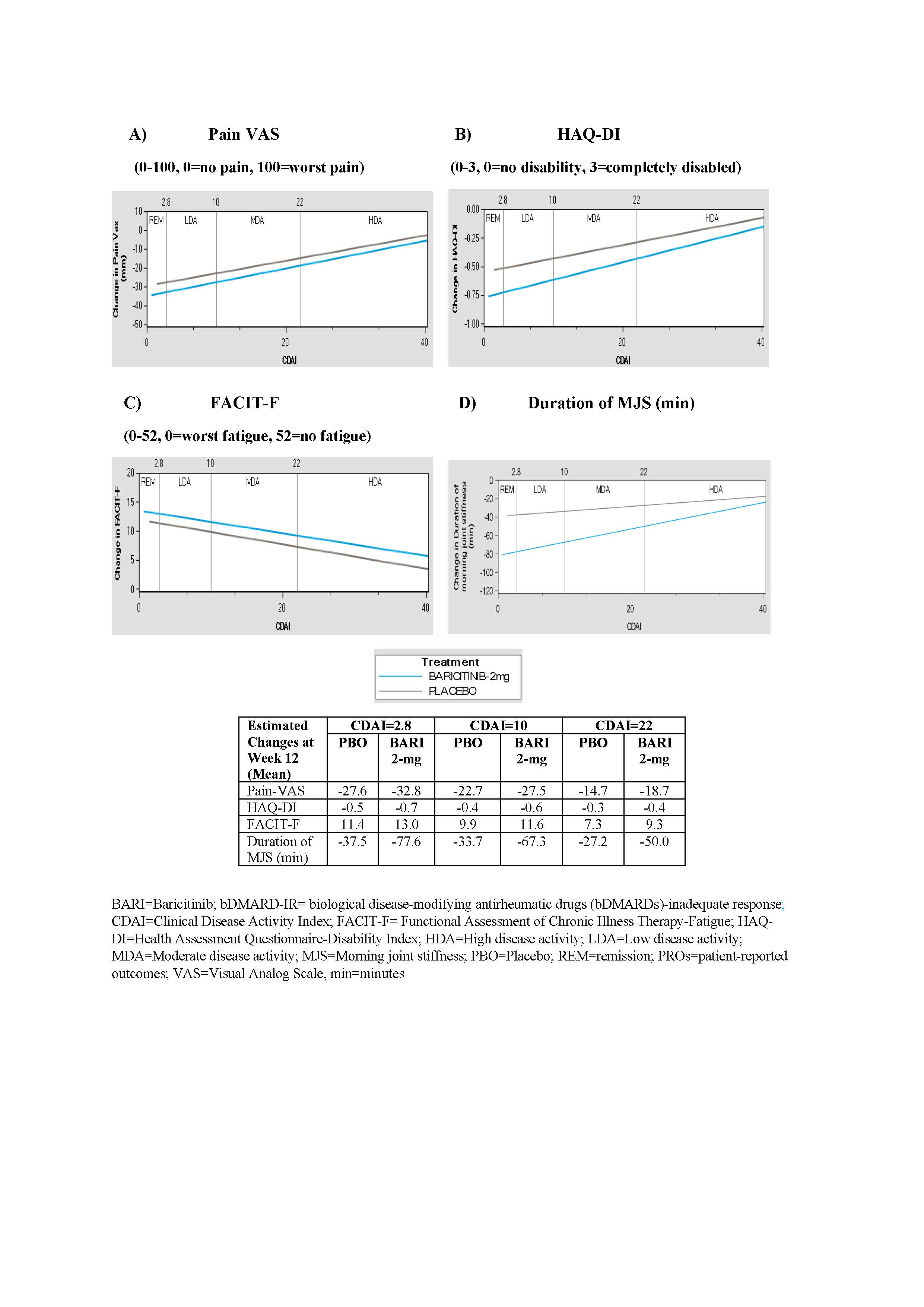 Figure 1: Estimated changes in PROs by CDAI disease activity at Week 12 in RA-BEACON (bDMARD-IR) trial. A) Pain VAS, B) HAQ-DI, C) FACIT-F, D) Duration of MJS.
Figure 1: Estimated changes in PROs by CDAI disease activity at Week 12 in RA-BEACON (bDMARD-IR) trial. A) Pain VAS, B) HAQ-DI, C) FACIT-F, D) Duration of MJS.
 Figure 2: Estimated changes in PROs by CDAI disease activity at Week 12 in RA-BUILD (csDMARD-IR) trial. A) Pain VAS, B) HAQ-DI, C) FACIT-F, D) Duration of MJS.
Figure 2: Estimated changes in PROs by CDAI disease activity at Week 12 in RA-BUILD (csDMARD-IR) trial. A) Pain VAS, B) HAQ-DI, C) FACIT-F, D) Duration of MJS.
To cite this abstract in AMA style:
Bingham III C, Jia B, Wu J, Quebe A, Kannowski C, Otawa S, Chen Y, Griffing K, He D, Sholter D. Baricitinib 2-mg Provides Greater Improvements in Patient-Reported Outcomes Across All Disease Activity Levels Compared to Placebo: Post-hoc Analyses of RA-BEACON and RA-BUILD Trials [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/baricitinib-2-mg-provides-greater-improvements-in-patient-reported-outcomes-across-all-disease-activity-levels-compared-to-placebo-post-hoc-analyses-of-ra-beacon-and-ra-build-trials/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/baricitinib-2-mg-provides-greater-improvements-in-patient-reported-outcomes-across-all-disease-activity-levels-compared-to-placebo-post-hoc-analyses-of-ra-beacon-and-ra-build-trials/
