Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Patients with LN who received obinutuzumab, a humanized type II anti-CD20 monoclonal antibody, with standard-of-care (MMF) immunosuppression (Phase II NOBILITY; NCT02550652; PMID 34615636) showed improved clinical responses through Week 104 versus those who received MMF alone. Sustained depletion up to Week 52 was associated with a better initial clinical response.1 This analysis aims to characterize subsequent B-cell recovery after the last dose of obinutuzumab in NOBILITY and its impact on later response and safety.
Methods: 125 patients with active Class III/IV LN receiving MMF and corticosteroids were randomized and received either obinutuzumab 1000 mg (n=63) or placebo (n=62) on Day 1 and Weeks 2, 24 and 26, and followed through Week 104 or to B-cell recovery, whichever was longer. Blood B cells were measured using both a TBNK cell assay with a LLoQ of 10 CD19+ cells/µL and a high-sensitivity minimal residual B-cell 1.1 assay with an LLoQ of 0.4 cells/µL. B-cell depletion was defined as ≤0.4 cells/µL, and recovery was defined as ≥20 cells/µL or the patient’s predose baseline, whichever was lower. Time to peripheral B-cell recovery after the last dose of obinutuzumab, the relationship between time to recovery and efficacy at Week 104, and safety throughout the main study and follow-up period were evaluated (SAE and infectious SAE rates, adjusted for patient-years [PY] at risk).
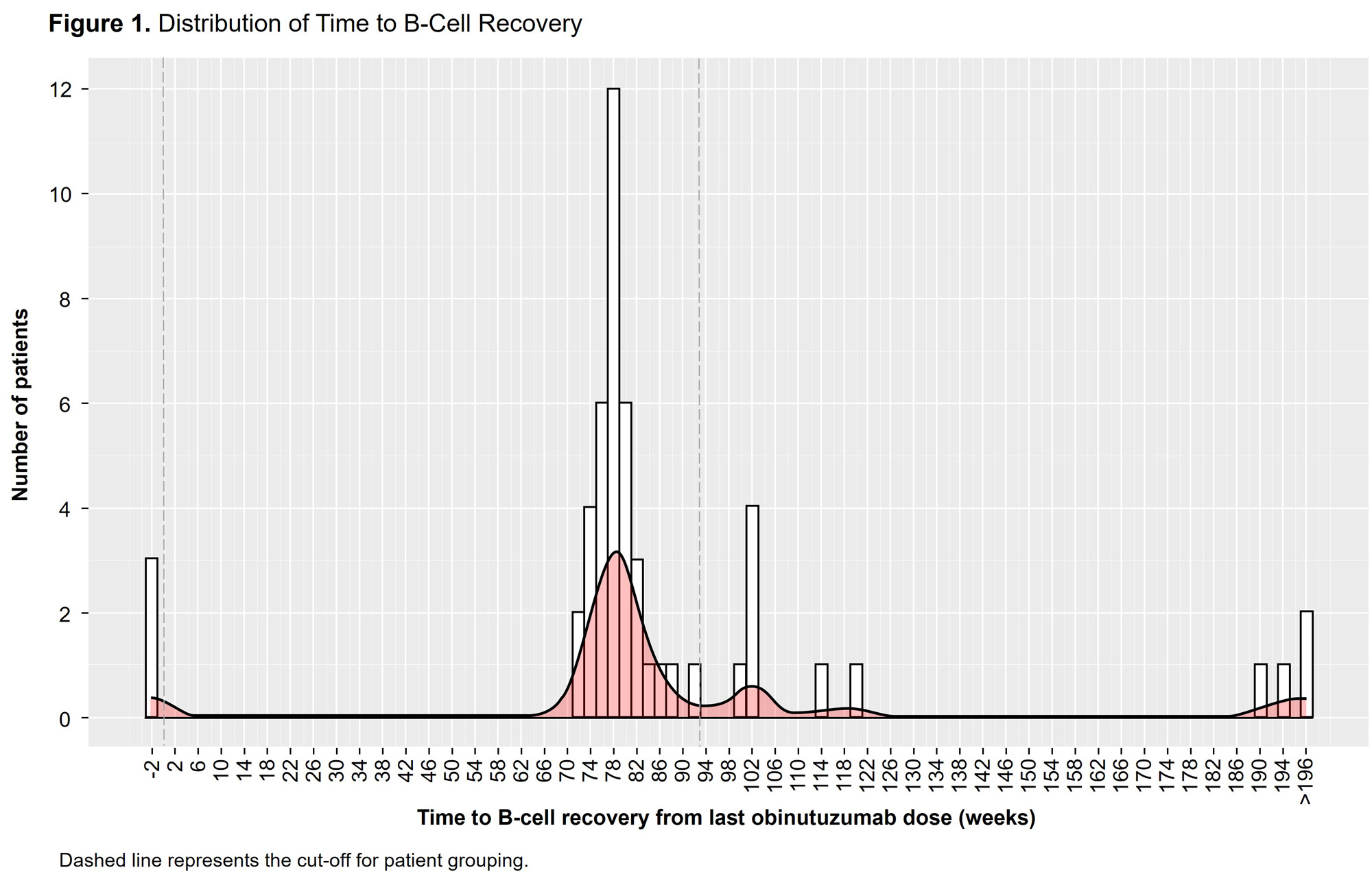
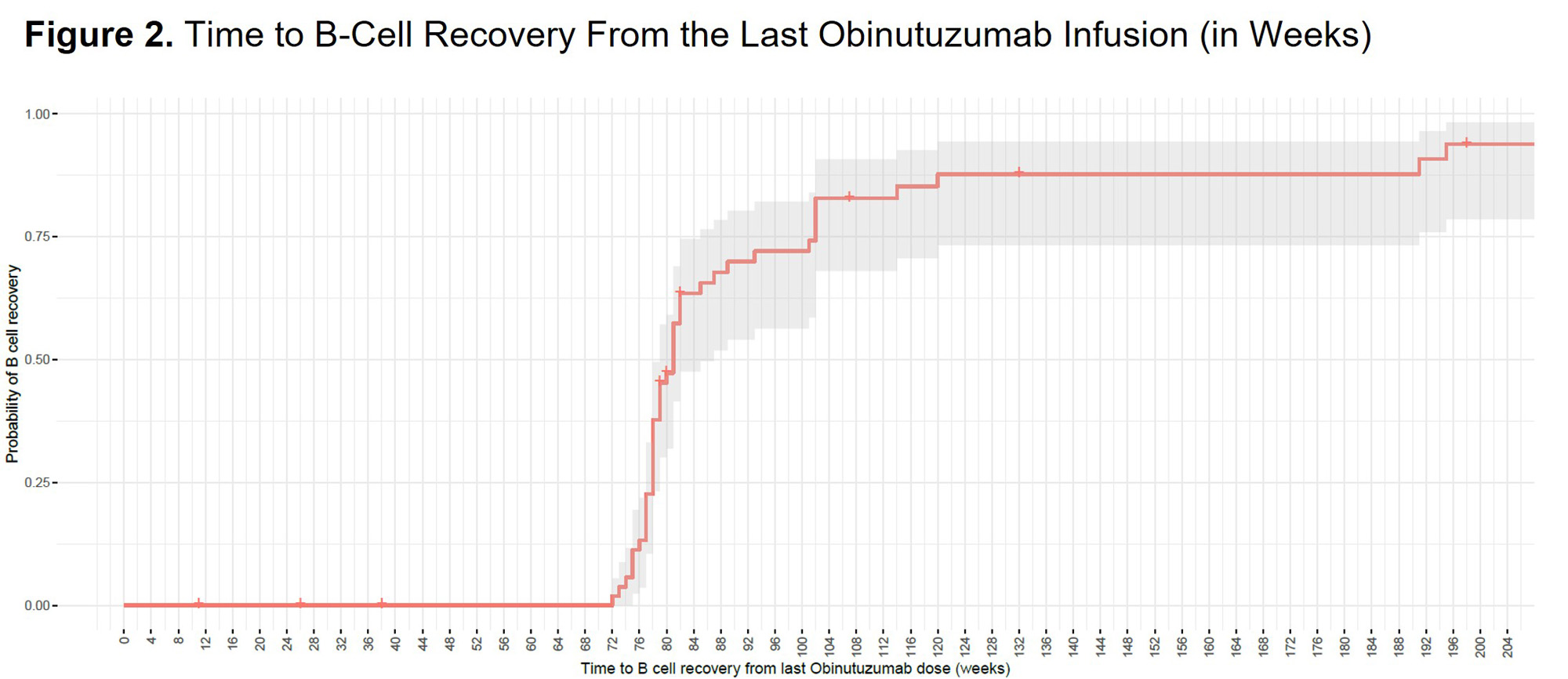
Results: Of 63 patients who received obinutuzumab, 4 did not achieve full B-cell depletion during the study. By Week 24, 59 patients (93.7%) achieved B-cell depletion (before obinutuzumab redosing); of those, 4 discontinued prior to B-cell recovery, and 4 completed the study at or after Week 104 but before achieving B-cell recovery. The remaining 51 patients comprised the analysis population, which was grouped based on the time to B-cell recovery distribution (Figures 1 and 2). Of the 51 patients, 3 (5.9%) recovered B cells before redosing at Week 26; 1/3 achieved CRR at Week 104. 37 patients (72.5%) attained B-cell recovery ≤93 weeks of their last dose of obinutuzumab (median, 78.1 weeks). 18/ 37 (48.6%) and 23/37 (62.2%) achieved CRR and overall renal response (ORR), respectively, at Week 104. In these 37 patients, SAE and infectious SAE rates per 100 PY were 13 and 8. 11 patients (21.6%) did not recover B cells by Week 93 after their last dose of obinutuzumab. 9/11 patients achieved B-cell recovery at a median of 102 weeks, and 2/11 had not yet achieved B-cell recovery at the time of writing. 5/11 patients (45.5%) achieved CRR, and 8/ 11 (72.7%) achieved ORR at Week 104. In these 11 patients, SAE and infectious SAE rates per 100 PY were 10 and 0, respectively.
Conclusion: Most patients recovered peripheral B cells ≤93 weeks after the last obinutuzumab dose. Renal response rates at Week 104 were similar among patients who recovered B cells within 2 years of their final obinutuzumab infusion and those who recovered later or were still depleted, suggesting a greater clinical effect of early sustained depletion vs duration of depletion on clinical response.1 Within the limitation of small sample size, the SAE and infectious SAE rates appeared similar regardless of the duration of B-cell depletion. 1. Vital E, et al. Arthritis Rheumatol. 2020;72 (suppl 10).
To cite this abstract in AMA style:
Vital E, Roccatello D, Black D, Jacob-Moffatt R, Looney C, Martins E, Mao H, Schindler T, Seghal H, Garg J, Ross Terres J, Furie R. B-Cell Recovery in a Randomized Controlled Trial of B-Cell Depletion with Obinutuzumab for the Treatment of Proliferative Lupus Nephritis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/b-cell-recovery-in-a-randomized-controlled-trial-of-b-cell-depletion-with-obinutuzumab-for-the-treatment-of-proliferative-lupus-nephritis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/b-cell-recovery-in-a-randomized-controlled-trial-of-b-cell-depletion-with-obinutuzumab-for-the-treatment-of-proliferative-lupus-nephritis/