Session Information
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: Rituximab (RTX) has previously been shown to impair antibody response to vaccines such as influenza and streptococcus. Recently, diminished COVID-19 vaccine responsiveness in RTX treated patients has been reported. While COVID-19 vaccination guidelines have adopted time since last RTX infusion as a consideration for likelihood of vaccine responsiveness, a retrospective study suggested that B Cell reconstitution may be a more accurate predictor of vaccine response. Our hypothesis was that B Cell reconstitution would be associated with vaccine responsiveness.
Methods: A retrospective chart review was performed to assess patients that visited a rheumatology practice from 2/24-5/20, 2021. Analysis included patients who had received treatment with RTX and had completed COVID-19 vaccination. Demographics, diagnoses, concurrent therapies, vaccine type/dates, time from last RTX to first vaccination quantitative serological vaccine response, and % CD19 positive cells in the lymphocyte population were collected. COVID-19 serological response was the primary outcome. Descriptive statistics and bivariate comparisons were performed. Negative and positive predictive value was calculated between COVID-19 serological response and B Cell reconstitution status.
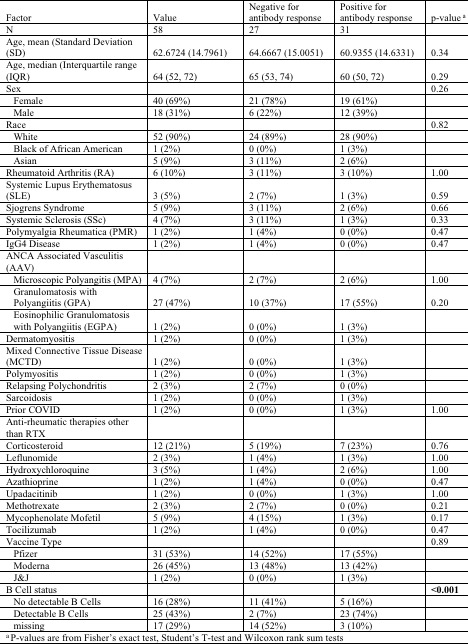
Results: 58 patients were identified who met criteria for inclusion. The majority were female (40, 69%), white (52, 90%), and the mean age was 62.67 years (Table 1).
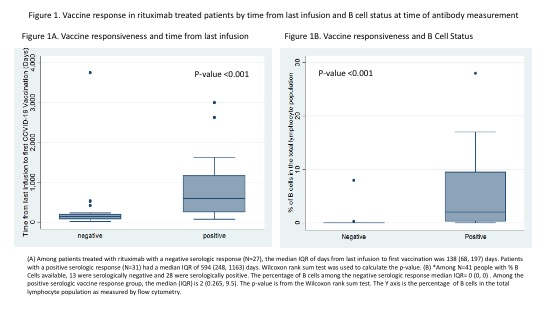
41 patients (71%) had B Cell status measured at time of antibody immunoassay. Time from last RTX was significantly longer in seropositive (median #days, IQR 594 (248, 1163) patients compared to seronegative patients (median #days, IQR 138 (68, 197) days) (p-value < 0.001). There was a significant difference in B Cell reconstitution between patients who had positive antibody responses to vaccination (median, IQR 2 (0.265-9.5) % of total lymphocyte population) versus negative responses (median, IQR 0 (0-0) % (p< 0.001) (Table 2). Only 12% (3/25) of patients >12 months since last RTX did not demonstrate a serologic responses, whereas 24% of patients (9/37) > 6 months since last RTX and 50% (6/12) of those 6-12 months from last RTX did not have a serologic response to the vaccine (Figure 1, Table 2).
B Cell reconstitution and time from last RTX were both significant indicators of vaccine response. (Figure 1). Seropositivity was 76% among those >6 months from last RTX exposure, however that increases to 92% in those with detectable B Cells (p=0.006). In patients 6-12 months from RTX exposure, only 50% were seropositive, however that increases to 83.33% in those with detectable B Cells (p=0.190) The positive predictive value of B Cell reconstitution for COVID-19 serologic response was 92% (95%CI: 74%-99%) and the negative predictive value was 68.8% (95%CI: 41.3%-89%).
Conclusion: B Cell reconstitution and longer time from last RTX were associated with a positive serologic response to the COVID-19 vaccine. A substantial number of patients >6 months from last RTX did not have a serologic response, and B cell reconstitution increased the likelihood of a response. Strategies for maximizing vaccine responsiveness in RTX treated patients should incorporate B Cell reconstitution and time from last RTX.
To cite this abstract in AMA style:
Jinich S, Jannat-Khah D, Spiera R. B Cell Reconstitution Is Strongly Associated with COVID-19 Vaccine Responsiveness in Rheumatic Disease Patients Treated with Rituximab [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/b-cell-reconstitution-is-strongly-associated-with-covid-19-vaccine-responsiveness-in-rheumatic-disease-patients-treated-with-rituximab/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/b-cell-reconstitution-is-strongly-associated-with-covid-19-vaccine-responsiveness-in-rheumatic-disease-patients-treated-with-rituximab/