Session Information
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: Systemic autoinflammatoy diseases (SAIDs) are primarily caused by abnormal innate immune response. NOD-like receptors (NLRs) are intracellular sensors to the immune process, including NOD2, pyrin, cryopyrin, and NLRP12. Next generation sequencing is used to test related genetic markers for diagnostic purpose. Digenic or oligogenic mutations are occasionally identified in individual patients causing difficult interpretation of their clinical significance. This study aimed to analyze the clinical, molecular, and therapeutic data of patients.
Methods: Electronic medical records of a cohort of patients with SAIDs between 2016 to April 2022 were reviewed. Patients were referred and managed by subspecilists in our Center of Autoinflammatory disease. Systemic autoimmune and other diseases were excluded after extensive workups. All patients underwent molecular testing for periodic fever syndrome 6-gene panel (MEFV, TNFRSF1A, NLRP3, MVK, NLRP12 and NOD2). An indivivual SAID was diagnosed based on characteristic phenotype and specific genotype. The study was approved by the Stony Brook University Institutional Research Board.
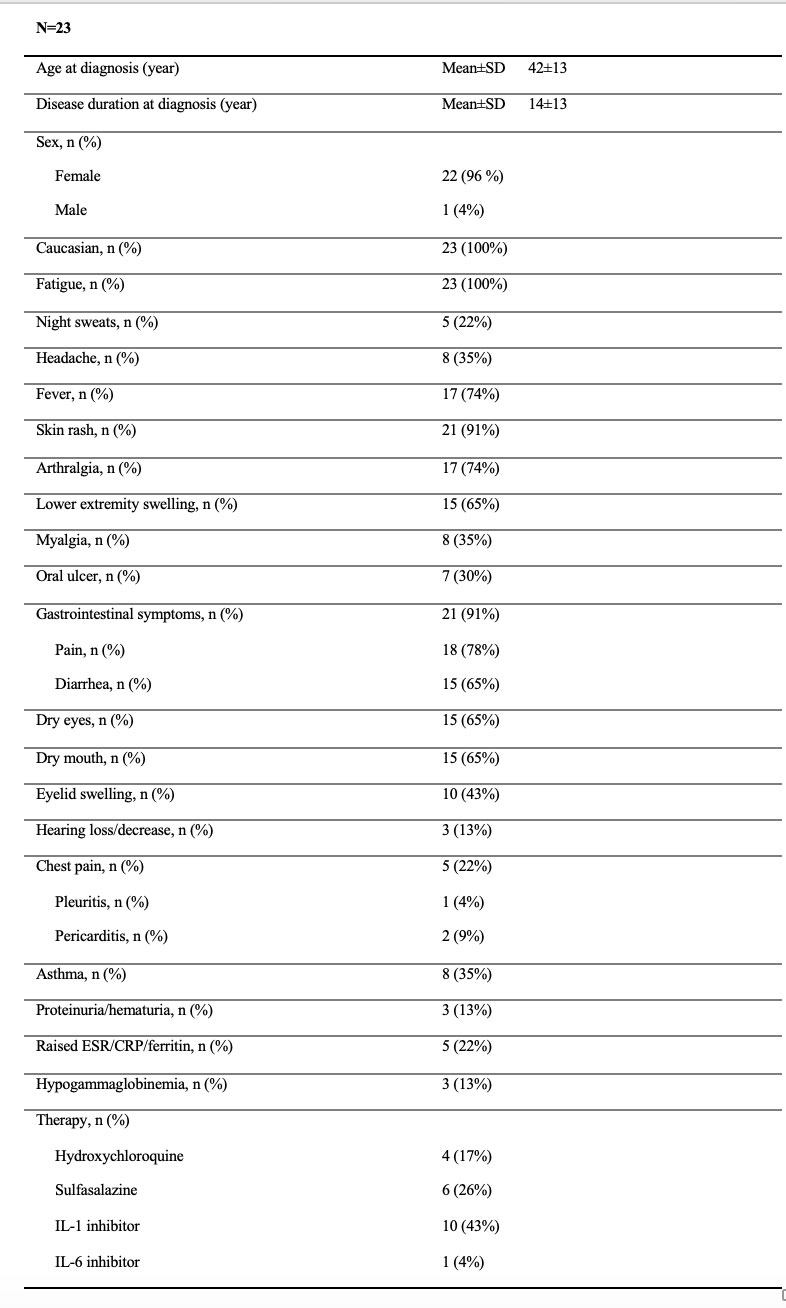
Results: A total of 23 adult patients with SAIDs were included. All patients were Caucasian with 96% females, the mean age was 42±13 years at diagnosis and disease duration was 14±13 years at diagnosis suggesting a prolonged diagnostic delay due to lack of recognition. Most patients had recurrent fever, rash, arthralgia/leg swelling, gastrointestinal and sicca-like symptoms/eyelid swelling (Table 1). Other symptoms included myalgia, oral ulcers, chest pain/pleuritic/pericarditis, asthma, and hearing loss. All patients carry NOD2 and other gene variants combinations, including NOD2/MEFV variants in 10 with poor response to colchicine mostly, NOD2/ NLRP12 in 6, NOD2/TNFRSF1A in 4 and NOD2/NLRP3 in 3(Table 2). Most variants are of low penetrance and are known to contribute to SAIDs. Due to the overlapping clinical phenotypes between SAIDs and carriage of multiple variants, accurate diagnosis is challenging. By close phenotype and genotype correlations, 11/23(48%) of patients were diagnosed with Yao Syndrome(YAOS, OMIM 617321), 2/23(9%) with atypical Familial Mediterranean Fever (FMF), and the remaining were suspected of having mixed diagnoses of two or more SAIDs such as YAOS, FMF, NLRP3-AID, NLRP12-AID, and TNF receptor associated periodic syndrome. Since all these genes encode NLRs except TNFRSF1A, and NOD2 was the denominator in our cohort, we propose to classify those patients having mixed NLR-associated autoinflammatory disease. Nearly half of our patients were treated with biologics, canakinumab mostly, suggesting that these patients would have more frequent disease flares. This may be supported by the literature data that multiple combined variants of small effect are known to cause equal to or even greater effects than a pathogenic variant of high penetrance.
Conclusion: This is the largest monocentric cohort of autoinflammatory disease patients with NOD2 and other coexisting variants. We provide our experience in the diagnosis, classification and management. We hope that our study may serve as preliminary guidance in dealing with this rare clinical scenario.
To cite this abstract in AMA style:
Nomani H, Navetta-Modrov B, Yun M, Yao Q. Autoinflammatory Diseases Associated with NOD2 and Other Concurrent Genetic Mutations [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/autoinflammatory-diseases-associated-with-nod2-and-other-concurrent-genetic-mutations/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/autoinflammatory-diseases-associated-with-nod2-and-other-concurrent-genetic-mutations/