Session Information
Date: Saturday, November 12, 2022
Title: SLE – Treatment Poster I
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
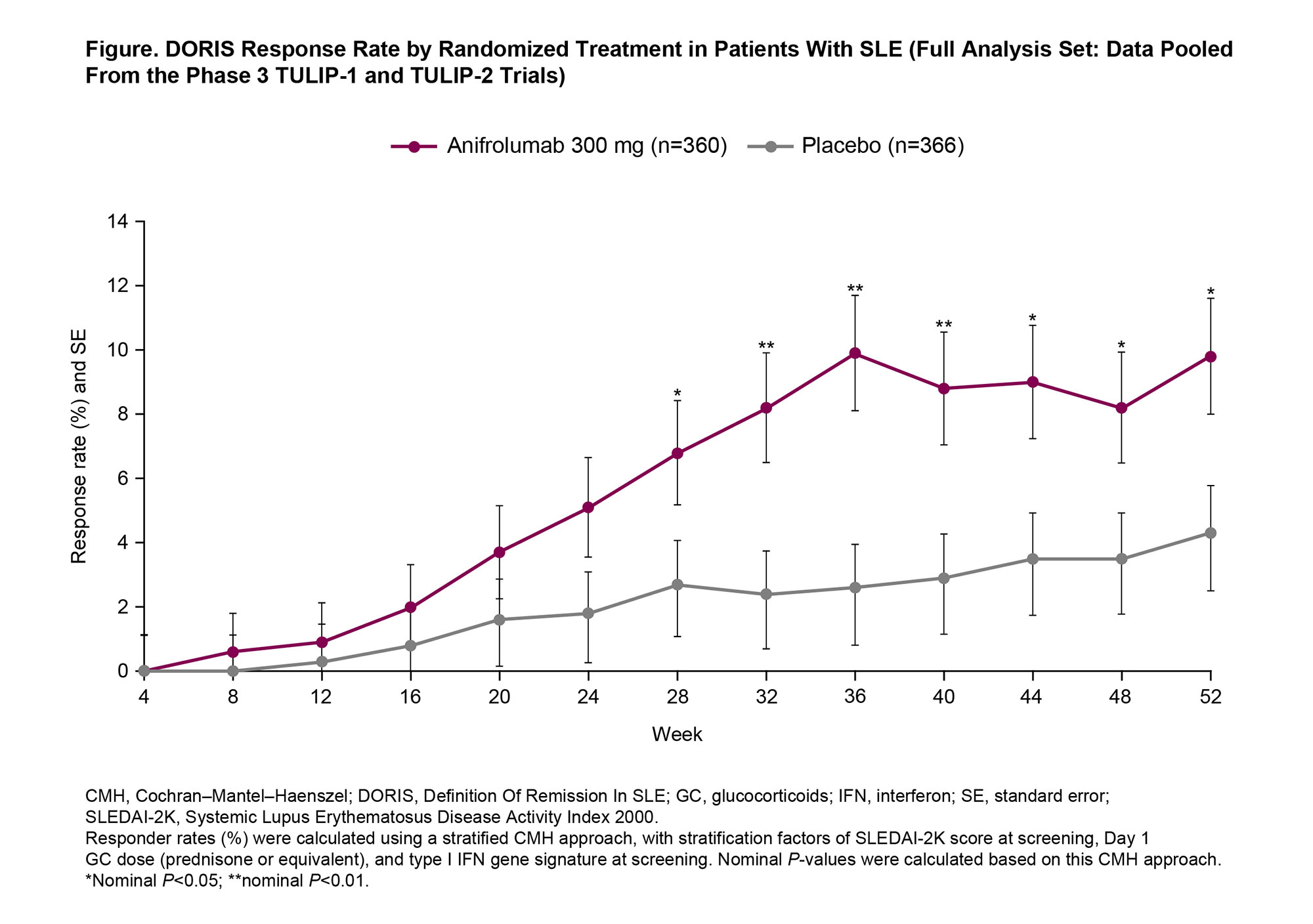
Background/Purpose: In patients with SLE, achieving remission is a treat-to-target goal. Remission is associated with lower rates of hospitalization and damage accrual and better quality of life, but is difficult to attain using the Definition of Remission In SLE (DORIS), as treatments to improve remission rates are limited.1 Using the DORIS,1 we investigated the frequency of remission with anifrolumab, a type I interferon receptor antibody, in the TULIP-1 (NCT02446912) and TULIP-2 (NCT02446899) trials.2,3
Methods: Pooled phase 3 data were analyzed in patients with moderate to severe SLE on standard of care who received intravenous anifrolumab 300 mg (n=360) or placebo (n=366) (monthly for 48 weeks) in the randomized, 52-week TULIP trials. All patients met the SLE 1997 ACR criteria. DORIS response was defined as clinical SLE Disease Activity Index 2000 (cSLEDAI-2K)=0, Physician’s Global Assessment (PGA)< 0.5 (0–3), prednisone or equivalent dose ≤5 mg/day, no use of restricted medications, and no premature discontinuation of study drug.1 Remission rate by timepoint, time to first remission, cumulative/percentage of time and proportion of consecutive visits in remission were compared between treatment groups using the Cochran–Mantel–Haenszel (CMH) approach, logistic regression, Cox regression, and analysis of covariance. All P-values are nominal.
Results: Baseline disease activity and SLE therapies were generally well balanced between the treatment groups. Remission rates generally increased over time, with nearly 10% of anifrolumab-treated patients (35/360) achieving remission between Weeks 36 and 52 compared with 3% for placebo. Remission was first achieved earlier in patients treated with anifrolumab 300 mg compared with placebo (Figure) (time to first remission, hazard ratio 2.4, 95% confidence interval [CI] 1.5–3.8, P< 0.001). The differences in remission rates based on the CMH analysis were higher in the anifrolumab 300-mg group vs placebo at all timepoints from Week 28 to Week 52 (all P< 0.05). More cumulative time (P< 0.001) and percentage of time (P< 0.001) were spent in remission by patients receiving anifrolumab than placebo, and cumulative time in remission thresholds of ≥20% (odds ratio [OR] 2.7, 95% CI 1.5–5.1, P=0.002) and ≥50% (OR 3.5, 95% CI 1.1–11.0, P=0.029) also favored anifrolumab. Anifrolumab-treated patients were more likely to be in sustained remission for ≥3 consecutive monthly visits (OR 2.5, 95% CI 1.4–4.4, P=0.003), ≥5 consecutive monthly visits (OR 3.1, 95% CI 1.3–7.0, P=0.008), or ≥7 consecutive monthly visits (OR 5.6, 95% CI 1.6–19.7, P=0.007).
Conclusion: Nearly 10% of anifrolumab-treated patients achieved remission, suggesting that DORIS response is an attainable outcome for patients with SLE treated with anifrolumab, and was associated with earlier, more frequent, more prolonged, and more sustained achievement of remission compared with placebo.
References:
- van Vollenhoven RF, et al. Lupus Sci Med. 2021;8:e000538.
- Morand EF, et al. N Engl J Med. 2020;382:211–21.
- Furie RA, et al. Lancet Rheumatol. 2019;1:e208–19.
To cite this abstract in AMA style:
Van Vollenhoven R, Morand E, Furie R, Bruce I, Abreu G, Tummala R, Al-Mossawi H, Lindholm C. Attainment of Remission with Anifrolumab: A Post Hoc Analysis of Pooled TULIP-1 and TULIP-2 Datasets [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/attainment-of-remission-with-anifrolumab-a-post-hoc-analysis-of-pooled-tulip-1-and-tulip-2-datasets/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/attainment-of-remission-with-anifrolumab-a-post-hoc-analysis-of-pooled-tulip-1-and-tulip-2-datasets/