Session Information
Date: Sunday, October 26, 2025
Title: (0001–0018) B Cell Biology & Targets in Autoimmune & Inflammatory Disease Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: CD19-targeted therapies, such as chimeric antigen receptor (CAR)-T or T-cell engagers (TCE), have been approved for the treatment of B cell malignancies. By depleting autoreactive B cells, CD19-targeted therapies have shown early yet promising efficacy in treating patients with B cell-driven autoimmune diseases. However, the clinical application of TCE continues to be greatly hindered by the unfavorable pharmacokinetics, incomplete B cell depletion and toxicity associated with cytokine release syndrome (CRS). Here we developed a “2+1” CD19 x CD3 TCE, ATG-201, for the treatment of B cell driven autoimmune diseases, which utilizes a steric hindrance-based masking technology and a novel fast-on-fast-off CD3 binder, and effectively depletes B cells with minimal risk of CRS.
Methods: ATG-201 was constructed by introducing a novel fast-on-fast-off, conformational epitope-targeted anti-CD3 single chain fragment variable (scFv) to the hinge region of one of the heavy chains of a CD19 monoclonal antibody. It was evaluated in a series of in vitro and in vivo studies for binding affinity, B cell depletion, cytokine release, anti-disease efficacy and drug developability. The safety and pharmacokinetic (PK) parameters of ATG-201 were evaluated in hCD19/hCD3 knock-in mice, and non-human primates (NHP) using a monkey surrogate TCE antibody.
Results: According to cryo-EM data, the CD3 binding site is masked by the constant region of the CD19-targeting Fab arm in the absence of CD19 cross-linking. ATG-201 showed limited CD3+ T cell binding before CD19-crosslinking (Figure 1). It activated T cells and only in the presence of CD19+ cells. ATG-201 demonstrated stronger B cell depletion activity in PBCMs derived from SLE patient and healthy donors with much lower cytokine release compared to benchmark TCEs in vitro (Figure 2). Single dose of ATG-201 completely and deeply depleted B cells in blood, bone marrow and spleen of CD34+ cells humanized NDG mice (Figure 3). The mouse surrogate CD19xCD3 TCE demonstrated potent efficacy in MOG-EAE model and MRL-lpr mouse SLE model, with deeper B cell depletion observed compared to CD20-targeted TCE. ATG-201 and its monkey surrogate CD19xCD3 TCE exhibited a favorable pharmacokinetic profile in hCD19/hCD3 knock-in mice and NHP respectively. ATG-201 was stable at high concentration (125mg/ml) and under stress conditions. ATG-201 and its monkey surrogate CD19xCD3 TCE were well tolerated in hCD19/hCD3 knock-in mice and NHP.
Conclusion: ATG-201 demonstrates CD19-dependent CD3 binding and activation, inducing effective B cell depletion and anti-disease activity in vitro and in vivo with good PK and safety profile, which warrants further clinical evaluation in the treatment of B cell related autoimmune diseases.
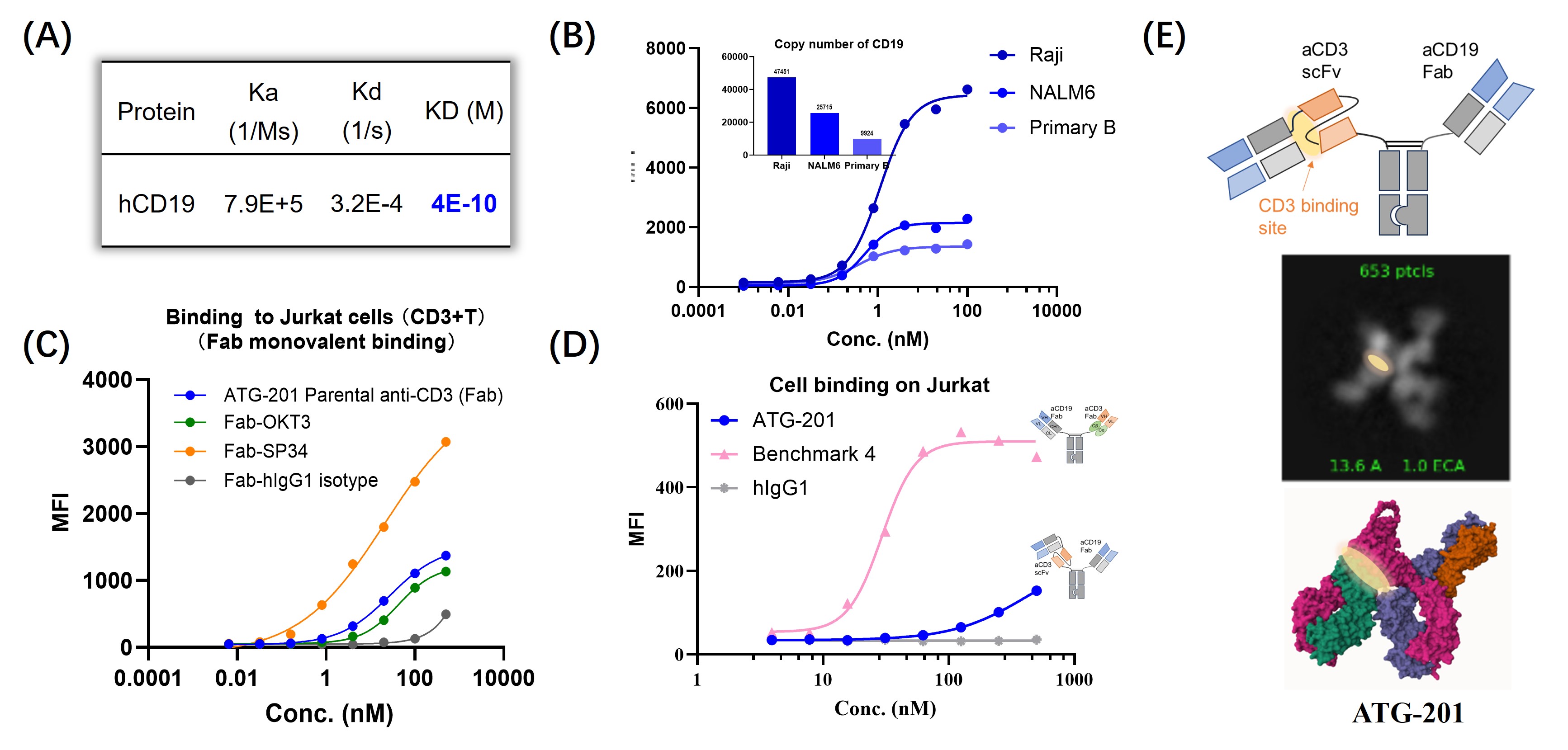 Figure 1. ATG-201 showed limited CD3+ T cell binding before CD19-crosslinking. (A) CD19 protein binding KD of ATG-201 detected by SPR. (B) Binding of ATG-201 to Raji, NALM6 and primary B cells. (C) Monovalent binding of ATG-201’s parental anti-CD3 Fab binder to CD3+T cell. (D) Binding of ATG-201 and BMK4 to T cells before CD19 crosslinking. (E) The CD3 binding site is masked by the constant region of the CD19-targeting Fab arm in the absence of CD19 crosslinking by cryo-EM analysis
Figure 1. ATG-201 showed limited CD3+ T cell binding before CD19-crosslinking. (A) CD19 protein binding KD of ATG-201 detected by SPR. (B) Binding of ATG-201 to Raji, NALM6 and primary B cells. (C) Monovalent binding of ATG-201’s parental anti-CD3 Fab binder to CD3+T cell. (D) Binding of ATG-201 and BMK4 to T cells before CD19 crosslinking. (E) The CD3 binding site is masked by the constant region of the CD19-targeting Fab arm in the absence of CD19 crosslinking by cryo-EM analysis
.jpg) Figure 2. ATG-201 induced enhanced naïve B cell depletion and reduced cytokine release compared to clinical benchmarks. B cell depletion (A, B) and concentration of the indicated cytokines in the supernatant (C, D) of isolated human PBMC incubated with ATG-201 or Benchmarks for 48 hours.
Figure 2. ATG-201 induced enhanced naïve B cell depletion and reduced cytokine release compared to clinical benchmarks. B cell depletion (A, B) and concentration of the indicated cytokines in the supernatant (C, D) of isolated human PBMC incubated with ATG-201 or Benchmarks for 48 hours.
.jpg) Figure 3. ATG-201 induces complete B cell depletion in CD34+ hematopoietic stem cells humanized mice with reduced cytokine release. CD34+cell humanized NDG mice were treated with a single dose of 1.5 mg/kg ATG-201 or Bechmark4 (Molar equivalent dose of 1.5 mpk ATG-201). (A) Blood, bone marrow and spleen were collected and analyzed for B cells counts by FACS 3 days (D3), 5 days (D5), 7days (D7), or 14 days (D14) post treatment. (B) Plasma cytokine concentration post treatment.
Figure 3. ATG-201 induces complete B cell depletion in CD34+ hematopoietic stem cells humanized mice with reduced cytokine release. CD34+cell humanized NDG mice were treated with a single dose of 1.5 mg/kg ATG-201 or Bechmark4 (Molar equivalent dose of 1.5 mpk ATG-201). (A) Blood, bone marrow and spleen were collected and analyzed for B cells counts by FACS 3 days (D3), 5 days (D5), 7days (D7), or 14 days (D14) post treatment. (B) Plasma cytokine concentration post treatment.
To cite this abstract in AMA style:
Bian G, Li T, Liu H, Hu Z, Chen P, Mei J, Hou B. ATG-201, a Novel Steric Hindrance-based Masking CD19xCD3 T-cell Engager (TCE) for the Treatment of B Cell-related Autoimmune Diseases [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/atg-201-a-novel-steric-hindrance-based-masking-cd19xcd3-t-cell-engager-tce-for-the-treatment-of-b-cell-related-autoimmune-diseases/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/atg-201-a-novel-steric-hindrance-based-masking-cd19xcd3-t-cell-engager-tce-for-the-treatment-of-b-cell-related-autoimmune-diseases/
